Paper is coming out today it seems:
Led by researchers at the Center for Infection and Immunity (CII) at Columbia University Mailman School of Public Health with a multicenter team of leading ME/CFS researchers, the new study reveals molecular-level details into the syndrome’s lasting effects on inflammation and immune response...
www.eurekalert.org
News Release 1-Sep-2025
Study Reveals Details of Overactive Immune System in Patients with Chronic Fatigue Syndrome (ME/CFS)
Peer-Reviewed Publication
Columbia University's Mailman School of Public Health
Patients with chronic fatigue syndrome/myalgic encephalomyelitis (ME/CFS) have heightened innate immune responses to bacteria, viruses and fungi. While these responses are essential to fight infection, they can cause damage when unchecked. Led by researchers at the Center for Infection and Immunity (CII) at Columbia University Mailman School of Public Health with a multicenter team of leading ME/CFS researchers, the new study reveals molecular-level details into the syndrome’s lasting effects on inflammation and immune response that could inform the development of targeted therapeutic interventions to reduce symptoms of ME/CFS and other postinfectious syndromes such as post-treatment Lyme disease and Long COVID. Study findings are published in the journal
npj Metabolic Health and Disease.
Symptoms of ME/CFS include unexplained fatigue, post-exertional malaise (PEM), and cognitive dysfunction. According to the Centers for Disease Control and Prevention, the United States alone has up to 3.3 million ME/CFS cases, and an annual economic burden of up to 51 billion dollars. Once thought to be a psychological disorder, there is abundant evidence from studies of blood, muscle and brain that ME/CFS is a physical syndrome. Most patients report an influenza-like illness prior to developing ME/CFS. Researchers have hypothesized that the condition is the result of an abnormal response to infection that results in inflammation and cellular damage that impairs energy metabolism. The overlap in symptoms between ME/CFS and Long COVID confirms that infection can trigger the syndrome.
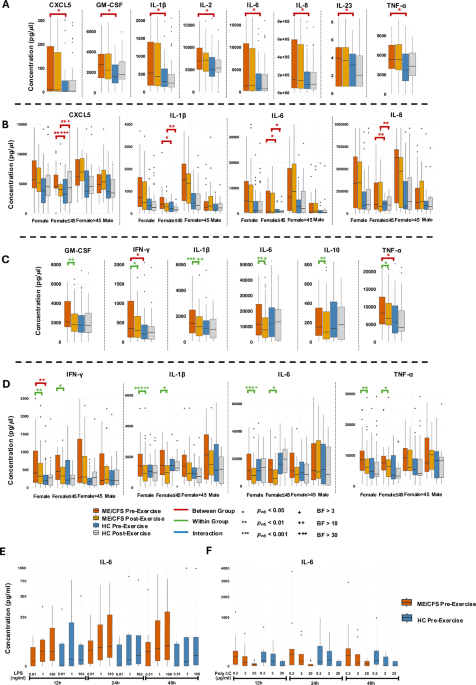
As part of the new study, researchers analyzed blood samples from 56 ME/CFS patients and 52 healthy controls recruited in New York and California. They used molecular testing to map the metabolome (metabolites of cellular metabolism) and proteome (proteins produced through biological processes). They also examined immune responses to microbial stimulation—a simulated infection—before and after exercise.
In ME/CFS patients, they observed disruptions in interconnected pathological processes that are often observed in chronic inflammatory conditions, suggesting a state of metabolic dysfunction, immune dysregulation, and tissue damage, potentially triggering a systemic inflammatory response. Understanding these relationships is crucial for developing therapeutic strategies targeting multiple aspects of the disease process.
- Impaired cellular energy production, which may lead to physical and mental exhaustion and the accumulation of toxic metabolites.
- Lipid abnormalities that contribute to tissue damage and perpetuate inflammation.
- Disruptions to the extracellular matrix which provides structural support and regulates cell behavior and release of signaling molecules that promote inflammation.
- Disruption of epithelial barriers, particularly in the gut which can contribute to the development of gut dysbiosis, where the balance of gut microbiota is altered resulting in the translocation of bacterial products into the blood where they trigger inflammation.
- Activation of the complement system of the innate immune system, the overactivation of which can contribute to tissue damage, inflammation, and sustained fatigue.
- Disturbances in copper-dependent antioxidant pathways, which can promote oxidative stress, promote inflammation, and contribute to tissue injury.
- Dysregulation of tryptophan-serotonin-kynurenine pathways, which can lead to impaired cognitive function.
In patients with ME/CFS, researchers observed that Peripheral Blood Mononuclear Cells (PBMCs)—blood cells that play a key role in the immune response—stimulated in a way to simulate infection using LPS (a bacterial product that activates immune responses) or poly I:C (a synthetic analogue of double-stranded RNA, mimicking viral infection) produced increased levels of IL-6, a cytokine central to inflammation. In addition, researchers used the TruCulture system to stimulate an immune response with
Staphylococcus enterotoxin B and HKCA (heat-killed
Candida albicans). The result was the release of higher levels of pro-inflammatory cytokines compared to healthy controls. These responses were higher in women than in men, with the highest levels in women over the age of 45 with lower levels of the sex hormones estradiol.
Clinical Trials and Targeted Therapies for ME/CFS Subtypes
The study’s authors write that their findings suggest potential candidates for clinical trials and targeted therapies. In addition to metformin—a medication primarily used to manage blood sugar levels in people with type 2 diabetes—the regulatory cytokine IL-37 and the mTOR inhibitor rapamycin, an immunosuppressive, may help ME/CFS individuals with evidence of enhanced innate immunity or hypersensitivity to microbial stimuli. Patients with evidence of an imbalanced gut microbiome may benefit from prebiotics (inulin) and probiotics (
F. prausnitzii) that enhance GI barrier integrity and regulate immune responses. Low baseline levels of 12,13-diHOME, a lipid molecule, and high post-exercise levels of GDF15, a hormone produced in response to various stressors that plays a role in regulating appetite and energy expenditure, may identify individuals with pronounced metabolic disruption who are more likely to respond to dietary supplementation with 12,13-diHOME or to treatment with a GDF15-neutralizing antibody. Individuals with abnormalities in metabolism of tryptophan, an essential amino acid, may respond to supplementation with 5-hydroxytryptophan or SSRIs. In patients with low levels of carnitine, a compound involved in metabolism, carnitine supplementation may restore carnitine shuttle function and enable use of lipids for energy source. Estrogen supplements may modulate the inflammatory response in older women with ME/CFS.
“Our findings indicate that people with ME/CFS have dysregulated immune responses to common infections” says Xiaoyu Che, PhD, co-first author. “These results suggest that specific intracellular pathways correlate with symptoms,” adds Amit Ranjan, PhD, co-first author.
“While what gives rise to ME/CFS remains obscure, understanding the ways it disrupts the body’s various biological processes on the molecular level is revealing biomarkers for specific ME/CFS subtypes that may inform clinical research and lead to targeted interventions,” says senior author W. Ian Lipkin, MD.
The authors are grateful for generous support from Hutchins Family Foundation through the Chronic Fatigue Initiative and NIH/NIAID (grant 4U54AI138370).
Xiaoyu Che, assistant professor of biostatistics in the CII, and Amit Ranjan, associate research scientist in the CII, are co-first authors. W. Ian Lipkin, John Snow Professor and director of the CII and the Center for Solutions for ME/CFS, is the study’s senior author. A complete list of authors is available in the journal article.
The authors declare no competing interests.
Journal
npj Metabolic Health and Disease
DOI
10.1038/s44324-025-00079-w
Method of Research
Observational study
Subject of Research
People
Article Title
Heightened innate immunity may trigger chronic in ammation, fatigue and post- exertional malaise in ME/CFS
Article Publication Date
1-Sep-2025
COI Statement
The authors declare no competing interests.