Ryan31337
Senior Member (Voting Rights)
Background
Postural orthostatic tachycardia syndrome (POTS) is a complex, multifaceted disorder that impairs functional status and quality of life. Current pharmacological treatments are limited.
Objectives
This study investigated the effect of ivabradine (selective blocker of the Ifunny channel in the sinoatrial node) on heart rate, quality of life (QOL), and plasma norepinephrine (NE) levels in patients with hyperadrenergic POTS defined by plasma NE >600 pg/ml and abnormal tilt table test.
Methods
In total, 22 patients with hyperadrenergic POTS as the predominant subtype completed a randomized, double-blinded, placebo-controlled, crossover trial with ivabradine. Patients were randomized to start either ivabradine or placebo for 1 month, and then were crossed over to the other treatment for 1 month. Heart rate, QOL, and plasma NE levels were measured at baseline and at the end of each treatment month.
Results
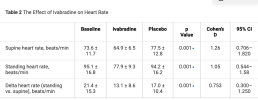
The average age was 33.9 ± 11.7 years, 95.5% were women (n = 21), and 86.4% were White (n = 23). There was a significant reduction in heart rate between placebo and ivabradine (p < 0.001). Patients reported significant improvements in QOL with RAND 36-Item Health Survey 1.0 for physical functioning (p = 0.008) and social functioning (p = 0.021). There was a strong trend in reduction of NE levels upon standing with ivabradine (p = 0.056). Patients did not experience any significant side-effects, such as bradycardia or hypotension, with ivabradine.
Conclusion
Ivabradine is safe and effective in significantly improving heart rate and QOL in patients with hyperadrenergic POTS as the predominant subtype.
Paywall, https://www.jacc.org/doi/full/10.1016/j.jacc.2020.12.029
Now free access (Oct 2025)
Last edited by a moderator: