Dolphin
Senior Member (Voting Rights)
Now published, see post #3
---------
https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5131664
Abstract
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex disorder with no known underlying mechanisms, diagnostic tools, or treatments.
Multiple areas of dysfunction have been extensively studied, but rarely examined together.
We recruited age- and sex-matched ME/CFS patients and healthy controls for a multi-modal study examining energy metabolism, immune profiles and plasma protein levels.
Elevated levels of adenosine monophosphate (AMP) were detected in both plasma and immune cells.
Additionally, immune cells showed higher levels of adenosine diphosphate (ADP) and a reduced adenosine triphosphate/adenosine diphosphate (ATP/ADP) ratio.
These findings imply decreased ATP generation and the presence of energy stress within the immune cell population.
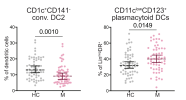
Adaptive immune cell populations were skewed towards less mature effector subsets of CD4+, CD8+ and gd T cells, and proportions of CD1c+CD141-conventional DC type 2 (cDC2) and CD56lowCD16+ terminal natural killer (NK) cells were also reduced.
Elevated levels of plasma proteins associated with thrombus formation and vascular reactivity may contribute to the endothelial dysfunction observed in ME/CFS patients.
Using Classification and Regression Tree (CART) modelling, we identified variables from each mode of investigation with strong predictive potential for ME/CFS.
Together, this study provides new insights into the somatic symptoms and underlying biology of ME/CFS.
---------
https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5131664
Mapping the Complexity of ME/CFS: Evidence for Abnormal Energy Metabolism, Altered Immune Profile and Vascular Dysfunction
Cell Reports Medicine
53 Pages Posted: 12 Feb 2025 Publication Status: Under Review
Ruiwen Benjamin Heng
Macquarie University
Bavani Gunasegaran
Macquarie University
Shivani Krishnamurthy
Macquarie University
Sonia Bustamante
University of New South Wales (UNSW)
Ananda Staats
Macquarie University
Sharron Chow
Macquarie University
Seong Beom Ahn
Macquarie University
Moumita Paul-Heng
The University of Sydney
Yolande Maciver
The Grove Health Pymble
Kirsten Smith
The Grove Health Pymble
Denise Phuong Tran
The University of Sydney
Peter P. Howley
Macquarie University
Ayse Aysin Bilgin
Macquarie University
Alexandra Sharland
The University of Sydney
Richard Schloeffel
Macquarie University
Gilles J. Guillemin
Macquarie University
Abstract
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex disorder with no known underlying mechanisms, diagnostic tools, or treatments.
Multiple areas of dysfunction have been extensively studied, but rarely examined together.
We recruited age- and sex-matched ME/CFS patients and healthy controls for a multi-modal study examining energy metabolism, immune profiles and plasma protein levels.
Elevated levels of adenosine monophosphate (AMP) were detected in both plasma and immune cells.
Additionally, immune cells showed higher levels of adenosine diphosphate (ADP) and a reduced adenosine triphosphate/adenosine diphosphate (ATP/ADP) ratio.
These findings imply decreased ATP generation and the presence of energy stress within the immune cell population.
Adaptive immune cell populations were skewed towards less mature effector subsets of CD4+, CD8+ and gd T cells, and proportions of CD1c+CD141-conventional DC type 2 (cDC2) and CD56lowCD16+ terminal natural killer (NK) cells were also reduced.
Elevated levels of plasma proteins associated with thrombus formation and vascular reactivity may contribute to the endothelial dysfunction observed in ME/CFS patients.
Using Classification and Regression Tree (CART) modelling, we identified variables from each mode of investigation with strong predictive potential for ME/CFS.
Together, this study provides new insights into the somatic symptoms and underlying biology of ME/CFS.
Note:
Funding Information: BG is supported by the Susie Myers Glioblastoma Scholarship (PANDIS) and Macquarie University Research Training Program Domestic Scholarship; SK is supported by International PhD scholarships from Macquarie University; MPH is supported by Sydney University Research Training Program Domestic Scholarship; SBA is supported by Cancer Council NSW funding RG23-06 and Targeted Call Research-National Health and Medical Research Council (NHMRC) funding GNT2015197; GJG was supported by the NHMRC funding APP1176660.
Declaration of Interests: The authors declare no competing interests
Last edited by a moderator: