Important to remember that - in this particularly chosen patient-cohort:
The patient study screening for acute EBV canditates, here; n=895. The recrutment stopped at 200 eligibiled canditates. VBB Wyller is obsessed that fatigue and pain after initial infection is sustained cuz the patients thinking, feeling and making stronger connetctions in the brain.
So to break this (fantasy - make believe - illusions hypothesis) signal-connections for fatigue and pain he initiated a early treatment program at 6 months (yes, same arguments for the Lightning Process (LP)). (and... ironyfact Herpes is persistent, once infected - they stays)
Adherens/satisfy to The Oxford criteria is bimodal CFQ ≥ 4 (as they say; severe fatigue).
How did it go - the results:
Adherens to ME/CFS criteria in this patient-cohort - baseline:

Fig:
6mo PI-EBV. VBB Wyller obtain/acquire the diagnostic criteria for ME/CFS by derive / extract post hoc or retrospectively, based on the answers to the participants / patients symptom questionnaire (yes, a non-specific method).
BASELINE: At 6-month PI-EBV, n = 91 participants were still fatigued (chronic fatigue (CF+); bimodal fatigue score CFQ value bimodal CFQ ≥ 4, while n = 104 had recovered from chronic fatigue (CF-); Bimodal fatigue score CFQ value ≤3. Which means that 53% of the patient population recovered from acute mono vs 47% of the participants still reported fatigue.
Of the 91 participants, 65 or 71.4% of the patients met only the Oxford 1991 criteria. n = 26 or 28.6% satisfied CDC1994 Fakuda criteria and n = 7 satisfied only Fakuda.
---
Baseline - RCT
Of all n= 43 participants, n=27 or as many as 62.8% of patients met only Oxford criteria. n = 16 or 37.2% satisfied CDC1994 Fakuda criteria and n = 6 or 37.5% satisfied only Fakuda. As many as 10 out of 16 Fakuda patients met the CCC2003 Canada criteria (62.5%), which for the entire patient cohort is reported 23.3%.
For the n= 21 participants in the mental therapy group (MT), 16 or as much as 76.2% of the patients met only the Oxford 1991 criteria. n=5 or 23.8% satisfied CDC1994 Fakuda criteria and n = 2 or 40% satisfied only Fakuda. As many as 3 out of 5 Fakuda patients met the CCC2003 Canada criteria (60%), and as reported for the entire patient group, 14.3%.
For the control group of n=22 patients (follow-up by a general practitioner; GP), n=11 or as many as 50% of the patients met only Oxford criteria. n =11 or 50% satisfied CDC1994 Fakuda criteria and n=4 or 42.9% satisfied only Fakuda. As many as 7 out of 11 Fakuda patients met the CCC2003 Canada criteria (57.1%), and as reported for the entire patient group, 31.8%.
When comparing the two groups; (MT n=16, 76.2%) vs (GP n=11, 50%) for how many only met Oxford criteria, shows that there are 18.5% and n = 5 more participants in the mental therapy arm than in the participant arm with general practitioner GP that only satisfies Oxford 1991 diagnostic criteria (CFQ ≥ 4).
It is not reported in the publication adheres to diagnostic criteria after 10 weeks of therapy treatment at 3 months primary endpoint (9 months PI-EBV) or another year later (21 months PI-EBV).
Oxford ONLY at Baseline: mental therapy group MT-arm 76.2% vs controllgr GP-arm 50%.
In the conclusion,
you assume correctly; more than 3/4 of the participants receiving CBT/Music - mental therapy treatment only suffered from fatigue with not even enough symptoms for stronger ME-criteria.
---
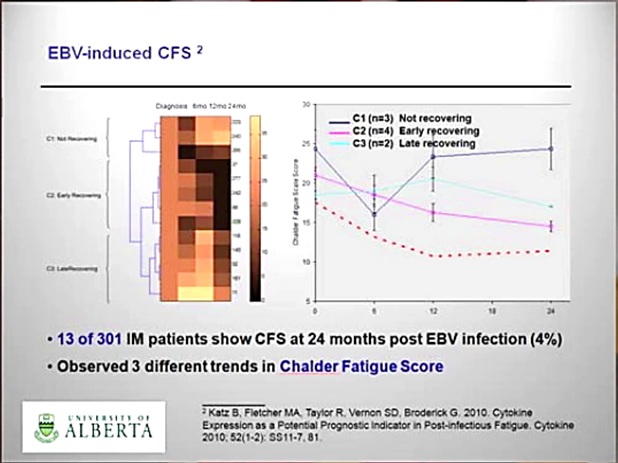
In the Katz et al study:
Katz et al. N=301 EBV; 6mo PI-EBV: n= 39 (12,9%), Female n= 35 (11,6%), Male n=4 (1,3%); 12mo PI-EBV: n= 22 (7,3%), F n=22 (7,3%), M n=0;
24 mo PI-EBV n= 13 (4,3%), F n=13 (4,3%), M n=0. ME-criteria; Jason
presentation
Gordon Broderick.
Only n=3 ME/CFS young girls showed a clinical immune signature compatible with ME.
Recovered from mono:

Malik et al_BMJ Paediatrics Open_utsnitt Tabell 4_Manuscript ID bmjpo-2019-000620
N=17 did NOT Recover from mono 21 months PI-EBV in Malik et al [
PMC old][
PMC new] but who the F**KK knows... only per-protocol data can answer that Q.
spontaneous or delayed recovery... vs treatmenteffect? per-protocol for MT arm at endpoint vs controlls has a statistical significance of p = 0.016 that mental therapy treatment makes patients activitylevels worse....
underpowered? well, find papers with CBT and GET arms...