- Home
- Forums
- Guidelines, reviews and disease coding
- ME/CFS related guidelines, reviews, disease coding
- Disease coding
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Updates on status of ICD-11 and changes to other classification and terminology systems
- Thread starter Dx Revision Watch
- Start date
Dx Revision Watch
Senior Member (Voting Rights)
So it seems like currently we're okay, but that if the 'evidence review' supports Dua's proposal, that could really screw everything up.
Indeed.
On March 17, 2017, Dr Robert Jakob, Team Leader, Classifications Terminologies and Standards, WHO, had written to me (and he had copied in seven other key WHO and ICD Revision personnel, including Dr Tarun Dua and the then ICD Revision Project Lead, Dr Ties Boerma):
"...As discussed earlier, chronic fatigue syndrome will not be lumped into the chapter ‘signs and symptoms’. We certainly will share the rationale for any decision."
Which could suggest a lack of consensus between Dr Jakob and Dr Dua/TAG Neurology.
WHO's Dr John Grove (current Project Lead) has stated (between Feb-March 2018):
that evidence is required for decisions towards allocating the relevant category in a better place in ICD-11 than the current one;
that a systematic review will determine if the category needs to be moved to any other specific chapter of ICD-11;
that the relevant category will in any case be kept separate from the generic ‘chronic fatigue’ (signs and symptoms);
that the outcomes will be posted together with the relevant detail on the proposal platform;
that new proposals posted on the platform will become part of the workflows of the maintenance mechanism of ICD-11 and be processed in an annual cycle; that results will be communicated, as soon as the involved committees have agreed on the recommendation on how to go about a specific proposal;
that the current updating cycle foresees a 3 yearly update to the classification structure, for the first update of ICD-11. Later updates to the classification structure may occur only at 5 yearly rates.
that a systematic review will determine if the category needs to be moved to any other specific chapter of ICD-11;
that the relevant category will in any case be kept separate from the generic ‘chronic fatigue’ (signs and symptoms);
that the outcomes will be posted together with the relevant detail on the proposal platform;
that new proposals posted on the platform will become part of the workflows of the maintenance mechanism of ICD-11 and be processed in an annual cycle; that results will be communicated, as soon as the involved committees have agreed on the recommendation on how to go about a specific proposal;
that the current updating cycle foresees a 3 yearly update to the classification structure, for the first update of ICD-11. Later updates to the classification structure may occur only at 5 yearly rates.
Edited to add: Also bear in mind the WHO/ICD-11 precedents for not relocating legacy terms to other chapters, as set out in early posts.
A few days before the "advance preview" was released, I counted over a 1000 proposals still to be processed. This backlog carries forward to become part of the update and maintenance tasks. With the working groups (TAGs) already sunsetted (October 2016) and the Joint Task Force due to step down this October, the review of existing proposals and new proposals becomes the responsibility of these committees:
Classifications and Statistics Advisory Commitee (CSAC)
- Includes experts from countries and from the groups listed below
Medical Scientific Advisory Committee (MSAC)
- Ensures scientific accuracy and clinical relevance of ICD
- Keeps links with the scientific community
Mortality Reference Group
- Advises on all aspects in relation to use of ICD in cause of death coding and rules
Morbidity Reference Group
- Advises on all aspects in relation to use of ICD in morbidity coding and rule
- Includes experts from countries and from the groups listed below
Medical Scientific Advisory Committee (MSAC)
- Ensures scientific accuracy and clinical relevance of ICD
- Keeps links with the scientific community
Mortality Reference Group
- Advises on all aspects in relation to use of ICD in cause of death coding and rules
Morbidity Reference Group
- Advises on all aspects in relation to use of ICD in morbidity coding and rule
So the joint proposal which I submitted with Mary Dimmock (in March 2017), the proposal submitted by Lily Chu on behalf of the IACFS/ME (in March 2017), the Dr Tarun Dua proposal (submitted November 2017), and any potential new proposals resulting out of the apparent "evidence review" or which might be submitted by any other party in the future, become the responsibility of the CSAC and MSAC.
The ICD-11 Reference Guide sets out the current projected update and maintenance schedule for ICD-11 and identifies what type of change would come under the heading of a "Minor change" and which would be considered a "Major change."
As far as I can determine, approval of a change of chapter or a change of parent class would likely come under "Major change." Which may mean that a change could not be implemented as part of the annual update schedule but would be rolled forward for batch incorporation in a 3 or 5 yearly update.
What isn't clear, yet, is in what years the first of the annual updates and the first of the 3 or 5 yearly updates would kick in.
The current Timeline is:
June 18, 2018: Release of an "advance preview" version of ICD-11 to allow Member States to prepare for implementation.
(Blue MMS Release 2018 remains stable.)
January 2019: ICD-11 submitted to the Executive Board Meeting.
May 2019: ICD-11 submitted to World Health Assembly for endorsement.
(Period during which still to be completed support materials and not yet published companion and derivative publications can be worked on.)
January 2022: WHA's endorsement comes into effect and Member States can start reporting using ICD-11 (if they have adopted and implemented the new edition by then. But few Members States are anticipated to be prepared at that point for migration to the new edition).
Some "Minor changes" are anticipated to be dealt with in between the annual update and maintenance process and if approved, entered into the orange Maintenance Platform until they can be incorporated into the next release of the blue MMS version. So the orange platform is a "work in progress" and won't have the stability of the blue MMS Release.
If at some point between now and 2022, the WHO were to submit new proposals on the Proposal Mechanism for the terms of interest to us, and those proposals were approved by the MSAC/CSAC, and if they constituted a "Major change", they might not be able to implement the change in the blue MMS Release until the first of the 3 or 5 yearly updates.
So we could be waiting a long time for stability.
Although I am retiring in August, I shall continue monitoring the Proposal Mechanism as I have agreed with Mary Dimmock that if we do need to respond to any new proposals, or to respond in relation to our joint proposal, that I will make myself available for this.
Last edited:
Thank you @Dx Revision Watch for your ongoing vigilence and attention to detail.
Dx Revision Watch
Senior Member (Voting Rights)
Thank you @Dx Revision Watch for your ongoing vigilence and attention to detail.
You're welcome, Amw66. I dream this stuff - I won't miss that.
Dx Revision Watch
Senior Member (Voting Rights)
By quietly postponing the date on which endorsement takes effect, the WHO gives itself an additional 3.5 yrs in which to finish off the ICD-11 package (including the companion publications and derivatives).
Prior to this June's release (and the updating of the Timeline page on June 14), there was no public indication, that I am aware of, that although presentation for WHA endorsement had been postponed, in January 2018, from May 2018 to May 2019 (and they had been discussing a postponement, at teleconferences, before Christmas, so I was aware of it back then) that WHA's May 2019 endorsement would not actually come into effect until January 2022.
Compare the January 2018 version of the Project Plan versus the Project Plan PDF, as it now stands:
http://www.who.int/classifications/icd/revision/icdprojectplan2015to2018.pdf
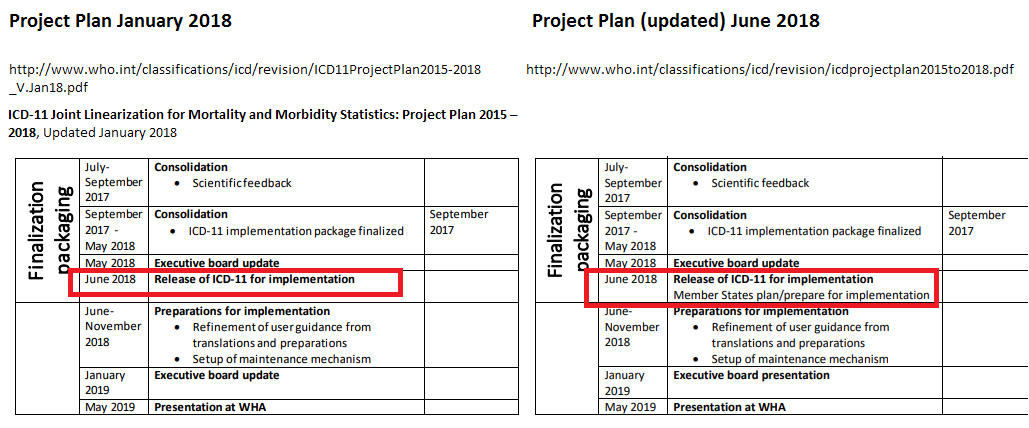
I used to pick up information from the WHO-FIC Network Council SEG meeting Agendas and Summary Reports. But no Reports from these meetings have been publicly posted since the Agenda for the April 2018 SEG teleconference.
Prior to this June's release (and the updating of the Timeline page on June 14), there was no public indication, that I am aware of, that although presentation for WHA endorsement had been postponed, in January 2018, from May 2018 to May 2019 (and they had been discussing a postponement, at teleconferences, before Christmas, so I was aware of it back then) that WHA's May 2019 endorsement would not actually come into effect until January 2022.
Compare the January 2018 version of the Project Plan versus the Project Plan PDF, as it now stands:
http://www.who.int/classifications/icd/revision/icdprojectplan2015to2018.pdf

I used to pick up information from the WHO-FIC Network Council SEG meeting Agendas and Summary Reports. But no Reports from these meetings have been publicly posted since the Agenda for the April 2018 SEG teleconference.
Last edited:
Inara
Senior Member (Voting Rights)
This is such a relief to know. THANK YOU!Although I am retiring in August, I shall continue monitoring the Proposal Mechanism as I have agreed with Mary Dimmock that if we do need to respond to any new proposals, or to respond in relation to our joint proposal, that I will make myself available for this.
At this time, both ME and CFS are included under "postviral fatigue syndrome" in the the neurological chapter in the version of ICD-11 released this past month. Neither term is under Bodily Distress Disorder, the term in the released version of ICD-11. @Dx Revision Watch can add more about what's happening with bodily distress syndrome
Dx Revision Watch
Senior Member (Voting Rights)
I am sorry for being thick but can you give me a summary as if I am a 2y old. So they have CFS under bodily distress syndrome? where is ME under?
For the post on ICD-11 and PVFS, BME and CFS, see Post #32:
https://www.s4me.info/threads/updat...nd-terminology-systems.3912/page-2#post-81626
Chapter 08: Diseases of the nervous system

Last edited:
Dx Revision Watch
Senior Member (Voting Rights)
For the post on ICD-11 and Bodily distress disorder, see Post #33:
https://www.s4me.info/threads/updat...nd-terminology-systems.3912/page-2#post-81630
Chapter 06: Mental, behavioural and neurodevelopmental disorders
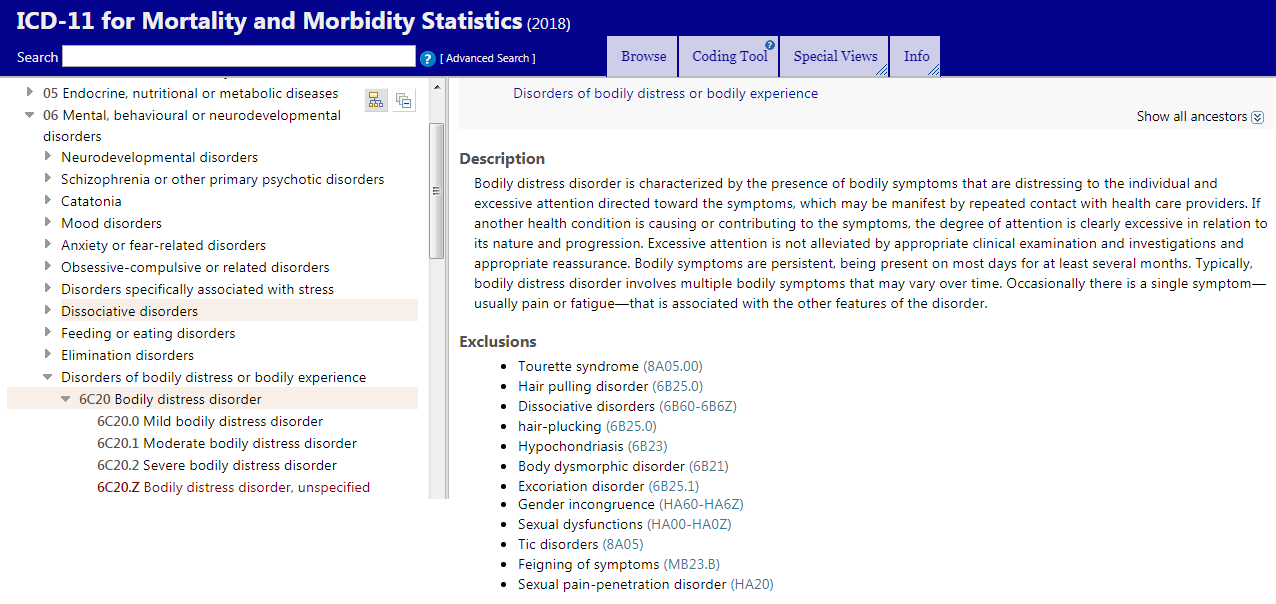
----------------------------------------------------------------------
For ease of reference for the three BDD severity specifiers: Mild BDD; Moderate BDD; Severe BDD:
(Note: DSM-5's "Somatic symptom disorder" is listed under Synonyms, under ICD-11's BDD.)

Note also:
Per Fink, 2017:

Budtz-Lilly, Fink et al outline some of the conceptual differences between SSD and BDS.
(Note these points are also applicable to ICD-11's BDD, which is closely aligned to DSM-5's SSD.)
Anna Budtz-Lilly, Per Fink, Eva Ornbol, Mogens Vestergaard, Grete Moth, Kaj Sparle Christensen, Marianne Rosendal. A new questionnaire to identify bodily distress in primary care: The ‘BDS checklist’. J Psychosom Res. June 2015 Volume 78, Issue 6, Pages 536–545
https://www.s4me.info/threads/updat...nd-terminology-systems.3912/page-2#post-81630
Chapter 06: Mental, behavioural and neurodevelopmental disorders

----------------------------------------------------------------------
For ease of reference for the three BDD severity specifiers: Mild BDD; Moderate BDD; Severe BDD:
(Note: DSM-5's "Somatic symptom disorder" is listed under Synonyms, under ICD-11's BDD.)

Note also:
Per Fink, 2017:

Budtz-Lilly, Fink et al outline some of the conceptual differences between SSD and BDS.
(Note these points are also applicable to ICD-11's BDD, which is closely aligned to DSM-5's SSD.)
“The newly introduced DSM-5 diagnosis, somatic symptom disorder (SSD), has replaced most of the DSM-IV somatoform disorder subcategories [10]. The diagnosis requires the presence of one or more bothering somatic symptoms of any aetiology and is not based on exclusion of any medical condition (…)
“BDS and SSD represent two very conceptually different diagnoses. BDS is based on symptom pattern recognition only, and symptoms are thought to be caused by hyperactivity in the central nervous system, whereas SSD criteria are based on prominent positive psycho-behavioural symptoms or characteristics, but no hypothesis of aetiology. BDS is assessed without asking patients about psychological symptoms.”
“BDS and SSD represent two very conceptually different diagnoses. BDS is based on symptom pattern recognition only, and symptoms are thought to be caused by hyperactivity in the central nervous system, whereas SSD criteria are based on prominent positive psycho-behavioural symptoms or characteristics, but no hypothesis of aetiology. BDS is assessed without asking patients about psychological symptoms.”
Anna Budtz-Lilly, Per Fink, Eva Ornbol, Mogens Vestergaard, Grete Moth, Kaj Sparle Christensen, Marianne Rosendal. A new questionnaire to identify bodily distress in primary care: The ‘BDS checklist’. J Psychosom Res. June 2015 Volume 78, Issue 6, Pages 536–545
Last edited:
Dx Revision Watch
Senior Member (Voting Rights)
I am sorry for being thick but can you give me a summary as if I am a 2y old. So they have CFS under bodily distress syndrome? where is ME under?
There is no "bodily distress syndrome" in the ICD-11 core edition.
There has never been a "bodily distress syndrome" proposed for the ICD-11 core edition.
The construct for ICD-11 is
Bodily distress disorder (BDD)
It is the case that the terms, "bodily distress disorder" and "bodily distress syndrome (BDS)" are often seen being used interchangeably in the literature when what is being referred to is Fink's BDS diagnostic construct/criteria, and Fink et al (2007, 2010) have themselves used the term "bodily distress disorder" for BDS and sometimes, "bodily distress syndrome/disorder."
As below, in this slide [2], and can also be seen in Fink et al papers:
Slide #10:
Extract:

But as defined and characterized for ICD-11, BDD has strong construct and criteria alignment with DSM-5's SSD and potentially captures the same patient population as SSD.
WHO's, Dr Geoffrey Reed (January 11, 2015):
"I agree that there is a potential for confusion with the Fink et al. construct, which is conceptually different. So, this is not ideal..."
Fink has also clarified in paper [1] and presentation [2] that the BDD disorder construct/criteria for the ICD-11 core edition (for which an advance pre-release version was published on June 18) is not his BDS construct/criteria.
"The DSM-5 somatic symptom disorder (SSD) is an example of a
diagnosis that is mainly based on psychological and behavioural
characteristics. The SSD pays very little attention to somatic symptoms;
only one disturbing somatic symptom of any aetiology - including
symptoms of cancer! - is required to qualify for criterion A of the diagnosis.
This will include most patients seen in both primary and secondary
care. The condition is defined by health anxiety, preoccupation
with symptoms or that the patient devotes excessive time and energy to
symptoms..."
"...The SSD supports the mind-body dichotomy as it is clearly defined
as a mental disorder, and it is of no differential diagnostic value for a
doctor in distinguishing between a well-defined medical condition and
bodily distress etc. The ICD-11 bodily distress disorder (BDD) draft
seems to be much in the line with the SSD thus having the same
problems [18]."
"...To include the BDS/BSD diagnosis and all the relevant functional
somatic syndromes in a separate chapter in the ICD-11 would have
been optimal, but the WHO has rejected this option."
"Unfortunately, the DSM-V classification and the ICD-11 psychiatric
draft rather seem to broaden the gap between medicine and psychiatry
instead of bridging it. The exclusive focus on the emotional and behavioural
symptoms in SSD and the BDD draft diagnosis may seem bizarre
and offending to the patients as these do not reflect the patients'
bothersome problem, namely bodily symptoms..."
diagnosis that is mainly based on psychological and behavioural
characteristics. The SSD pays very little attention to somatic symptoms;
only one disturbing somatic symptom of any aetiology - including
symptoms of cancer! - is required to qualify for criterion A of the diagnosis.
This will include most patients seen in both primary and secondary
care. The condition is defined by health anxiety, preoccupation
with symptoms or that the patient devotes excessive time and energy to
symptoms..."
"...The SSD supports the mind-body dichotomy as it is clearly defined
as a mental disorder, and it is of no differential diagnostic value for a
doctor in distinguishing between a well-defined medical condition and
bodily distress etc. The ICD-11 bodily distress disorder (BDD) draft
seems to be much in the line with the SSD thus having the same
problems [18]."
"...To include the BDS/BSD diagnosis and all the relevant functional
somatic syndromes in a separate chapter in the ICD-11 would have
been optimal, but the WHO has rejected this option."
"Unfortunately, the DSM-V classification and the ICD-11 psychiatric
draft rather seem to broaden the gap between medicine and psychiatry
instead of bridging it. The exclusive focus on the emotional and behavioural
symptoms in SSD and the BDD draft diagnosis may seem bizarre
and offending to the patients as these do not reflect the patients'
bothersome problem, namely bodily symptoms..."
For ICD-11 core edition, the disorder construct is the SSD-like, BDD, with Somatic symptom disorder under Synonyms to BDD.
1 Syndromes of bodily distress or functional somatic syndromes - Where are we heading. Lecture on the occasion of receiving the Alison Creed award 2017 Fink, Per Journal of Psychosomatic Research , Volume 97 , 127 - 130
(Paywalled) https://www.jpsychores.com/article/S0022-3999(17)30445-2/fulltext
2 Slides from: Lecture on the occasion of receiving the Alison Creed award 2017
http://www.eapm2017.com/images/site/abstracts/PLENARY_Prof_FINK.pdf
Last edited:
Dx Revision Watch
Senior Member (Voting Rights)
New document:
Comparison of SSD, BDD, BDS, BSS in classification systems
Version 1 | July 2018
https://dxrevisionwatch.files.wordp...-bdd-bds-bss-in-classification-systems-v1.pdf
or
http://bit.ly/2NmFZRa
Mary Dimmock and I have prepared a new Table and Notes comparing the key features of SSD, BDD, BSD and BSS.
At the top of the Table is a brief statement clarifying that ICD-11's BDD is differently conceptualized to Fink's BDS and captures different patient groups.
A copy of the PDF is also attached to this post.
We are still seeing confusion between ICD-11's BDD and Fink's BDS. Please share this document or the links on any platforms where members will find this comparison table helpful.
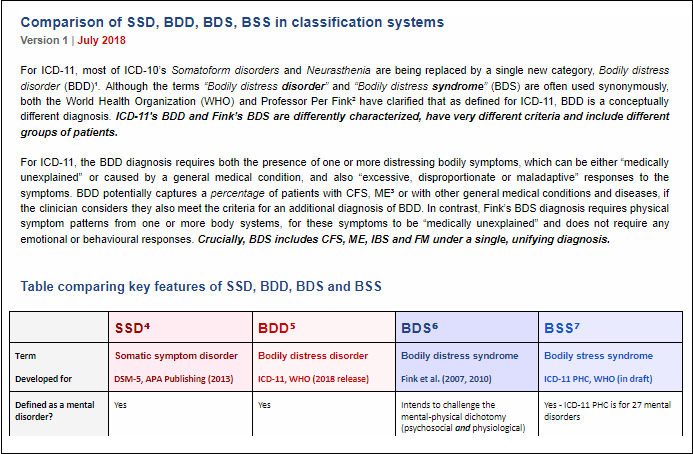
Comparison of SSD, BDD, BDS, BSS in classification systems
Version 1 | July 2018
https://dxrevisionwatch.files.wordp...-bdd-bds-bss-in-classification-systems-v1.pdf
or
http://bit.ly/2NmFZRa
Mary Dimmock and I have prepared a new Table and Notes comparing the key features of SSD, BDD, BSD and BSS.
At the top of the Table is a brief statement clarifying that ICD-11's BDD is differently conceptualized to Fink's BDS and captures different patient groups.
A copy of the PDF is also attached to this post.
We are still seeing confusion between ICD-11's BDD and Fink's BDS. Please share this document or the links on any platforms where members will find this comparison table helpful.

Attachments
Last edited:
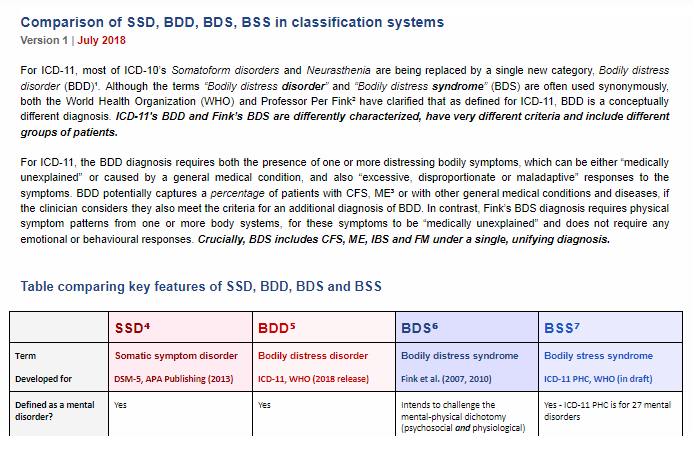
@Emsho background to NHS comments on petition?New document:
Comparison of SSD, BDD, BDS, BSS in classification systems
Version 1 | July 2018
https://dxrevisionwatch.files.wordp...-bdd-bds-bss-in-classification-systems-v1.pdf
or
http://bit.ly/2NmFZRa
Mary Dimmock and I have prepared a new Table and Notes comparing the key features of SSD, BDD, BSD and BSS.
At the top of the Table is a brief statement clarifying that ICD-11's BDD is differently conceptualized to Fink's BDS and captures different patient groups.
A copy of the PDF is also attached to this post.
We are still seeing confusion between ICD-11's BDD and Fink's BDS. Please share this document or the links on any platforms where members will find this comparison table helpful.
Dx Revision Watch
Senior Member (Voting Rights)
The document:
Comparison of Classification and Terminology Systems
has been updated with an amendment.
If you downloaded the document in Post #1 or Post #57 please replace the PDF with the most recent version:
Comparison of Classification and Terminology Systems
Version 3 | July 2018
https://dxrevisionwatch.files.wordp...lassification-and-terminology-systems-v-3.pdf
or
http://bit.ly/2JGUQUi
A PDF of Version 3 is also attached to this post.
Comparison of Classification and Terminology Systems
has been updated with an amendment.
If you downloaded the document in Post #1 or Post #57 please replace the PDF with the most recent version:
Comparison of Classification and Terminology Systems
Version 3 | July 2018
https://dxrevisionwatch.files.wordp...lassification-and-terminology-systems-v-3.pdf
or
http://bit.ly/2JGUQUi
A PDF of Version 3 is also attached to this post.
Attachments
Last edited:
A sincere thank you for your years of persistent and meticulous advocacy, @Dx Revision Watch. I was very sorry to read that you're retiring from advocacy, and of the way in which recent events have played out. I wish you and your family all the very best.
Dx Revision Watch
Senior Member (Voting Rights)
Audio file of WHO Press Conference
June 14, 2018 WHO Press Conference
14 June 2018 15:00 - 15:45
Release of ICD-11 – the 11th revision of the International Classification of Disease – Monday, 18 June 2018.
Mp3 audio file [39:25 min] from WHO Press Conference - launch of ICD-11 "advance release":
http://terrance.who.int/mediacentre/presser/WHO-RUSH_ICD-11_Virtual_Press_Conference_18JUN2018.mp3
June 14, 2018 WHO Press Conference
14 June 2018 15:00 - 15:45
Release of ICD-11 – the 11th revision of the International Classification of Disease – Monday, 18 June 2018.
- Dr Shekhar Saxena, Director, Department for Mental Health and Substance Abuse, WHO
- Dr Robert Jakob, Team Lead, Classifications, Terminologies and Standards, WHO
Mp3 audio file [39:25 min] from WHO Press Conference - launch of ICD-11 "advance release":
http://terrance.who.int/mediacentre/presser/WHO-RUSH_ICD-11_Virtual_Press_Conference_18JUN2018.mp3
Dx Revision Watch
Senior Member (Voting Rights)
Dr First notes in this recent presentation that ICD-11 uses stab point "clinical descriptions" rather than more rigid criteria sets, as used in DSM-5; that as a result, the clinical descriptions in the ICD-11 specialty publication, ICD-11 Clinical Descriptions and Diagnostic Guideline for Mental and Behavioural Disorders (the equivalent to ICD-10's "Blue Book") are more flexible than the DSM-5 code sets; that on the whole, disorder durations are less specific for ICD-11 than for DSM-5, and that clinical judgment may be used in ICD-11 if symptom duration is longer or shorter than specified.
[32:38 mins]
Michael First: Differences Between ICD-11 Classification of Mental & Behavioural Disorders and DSM-5
https://rop.no/roptv/hva-er-forskjellene-mellom-psykiske-lidelser-i-icd-11-og-dsm-5/
Published on 20 Jul 2018
First is a professor of clinical psychiatry, Columbia University, and research psychiatrist, New York State Psychiatric Institute. Dr. First is the Editorial and Coding Consultant for the DSM-5, the chief technical and editorial consultant on the World Health Organization's ICD-11, and is an external consultant to the NIMH Research Domain Criteria project. He was the Editor of the DSM-IV-TR, the Editor of Text and Criteria for DSM-IV and the APA's Handbook on Psychiatric Measures. In this lecture he gives an introduction to the differences between ICD-11 Classification of Mental and Behavioural Disorders and the DSM-5. The video was recorded in July 2018 for the Norwegian National Advisory Unit on Concurrent Substance Abuse and Mental Health Disorders (norwegian: Nasjonal kompetansetjeneste ROP: rop.no).
[32:38 mins]
Michael First: Differences Between ICD-11 Classification of Mental & Behavioural Disorders and DSM-5
https://rop.no/roptv/hva-er-forskjellene-mellom-psykiske-lidelser-i-icd-11-og-dsm-5/
Published on 20 Jul 2018
First is a professor of clinical psychiatry, Columbia University, and research psychiatrist, New York State Psychiatric Institute. Dr. First is the Editorial and Coding Consultant for the DSM-5, the chief technical and editorial consultant on the World Health Organization's ICD-11, and is an external consultant to the NIMH Research Domain Criteria project. He was the Editor of the DSM-IV-TR, the Editor of Text and Criteria for DSM-IV and the APA's Handbook on Psychiatric Measures. In this lecture he gives an introduction to the differences between ICD-11 Classification of Mental and Behavioural Disorders and the DSM-5. The video was recorded in July 2018 for the Norwegian National Advisory Unit on Concurrent Substance Abuse and Mental Health Disorders (norwegian: Nasjonal kompetansetjeneste ROP: rop.no).
Last edited:
Dx Revision Watch
Senior Member (Voting Rights)
https://hscic.kahootz.com/connect.ti/t_c_home/viewBlogArticle?articleID=261977&nextURL=/connect.ti/t_c_home/viewblog?blogid=50136
NHS Digital
ICD-11 Launch
"...No decision has been made for the implementation of ICD-11 in England, however NHS Digital plan to undertake further testing of the latest release and supporting products that will inform a future decision."
NHS Digital
ICD-11 Launch
"...No decision has been made for the implementation of ICD-11 in England, however NHS Digital plan to undertake further testing of the latest release and supporting products that will inform a future decision."
Melanie
Senior Member (Voting Rights)
@Dx Revision Watch I went through and liked all your posts forgetting you are going to get a bunch of "like" notifications. Anyway, thanks for all your hard work.
Anyway, thanks for all your hard work. 
Dx Revision Watch
Senior Member (Voting Rights)
According to this October 25-28, 2018 congress Abstract:
"As of the time of the Congress, the complete ICD-11 Clinical Descriptions and Diagnostic Guidelines (CDDG) will be available for public review and comment and a rigorous program of field testing will have been completed."
Edited to add:
But they had also said this in November 2017 ( http://www.aepc.es/PsClinicaX/CV/CVC4.html )
so Dr Reed might be referring not to a forthcoming public stakeholder review of the CDDG draft but to the GCPNetwork for clinicians who have been able to register for access to review and comment on the draft for a number of years.
http://www.aepc.es/PsClinicaXI/CV/CV_3.html
11th International Congress And 16th National Of Clinical Psychology, 25-28 October 2018, Granada (Spain)
Clinical descriptions and diagnostic guidelines of the ICD-11
Geoffrey Reed
Senior Project Officer of ICD-11 Mental and Behavioural Disorders
World Health Organization (WHO)
SWITZERLAND
1 English
Senior Project Officer of ICD-11 Mental and Behavioural Disorders, Department of Mental Health and Substance Abuse, World Health Organization...
(...)
KEYNOTE ABSTRACT
Clinical descriptions and diagnostic guidelines of the ICD-11
A decade of work by the World Health Organization Department of Mental Health and Substance Abuse to develop the ICD-11 classification of Mental and Behavioural Disorders is nearing completion. As of the time of the Congress, the complete ICD-11 Clinical Descriptions and Diagnostic Guidelines (CDDG) will be available for public review and comment and a rigorous program of field testing will have been completed. This address will provide an overview of WHO's work in developing the CDDG and the major features of the new classification...
"As of the time of the Congress, the complete ICD-11 Clinical Descriptions and Diagnostic Guidelines (CDDG) will be available for public review and comment and a rigorous program of field testing will have been completed."
Edited to add:
But they had also said this in November 2017 ( http://www.aepc.es/PsClinicaX/CV/CVC4.html )
so Dr Reed might be referring not to a forthcoming public stakeholder review of the CDDG draft but to the GCPNetwork for clinicians who have been able to register for access to review and comment on the draft for a number of years.
http://www.aepc.es/PsClinicaXI/CV/CV_3.html
11th International Congress And 16th National Of Clinical Psychology, 25-28 October 2018, Granada (Spain)
Clinical descriptions and diagnostic guidelines of the ICD-11
Geoffrey Reed
Senior Project Officer of ICD-11 Mental and Behavioural Disorders
World Health Organization (WHO)
SWITZERLAND
1 English
Senior Project Officer of ICD-11 Mental and Behavioural Disorders, Department of Mental Health and Substance Abuse, World Health Organization...
(...)
KEYNOTE ABSTRACT
Clinical descriptions and diagnostic guidelines of the ICD-11
A decade of work by the World Health Organization Department of Mental Health and Substance Abuse to develop the ICD-11 classification of Mental and Behavioural Disorders is nearing completion. As of the time of the Congress, the complete ICD-11 Clinical Descriptions and Diagnostic Guidelines (CDDG) will be available for public review and comment and a rigorous program of field testing will have been completed. This address will provide an overview of WHO's work in developing the CDDG and the major features of the new classification...
Last edited:
