rvallee
Senior Member (Voting Rights)
Persistent complement dysregulation with signs of thromboinflammation in active Long Covid
Open access: https://www.science.org/doi/10.1126/science.adg7942
Editor’s summary
Some individuals can endure persistent, debilitating symptoms for many months after an initial severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. However, the factors underpinning these health issues, called Long Covid, are poorly understood. Comparing the blood of patients with confirmed SARS-CoV-2 infection with that of uninfected controls, Cervia-Hasler et al. found that patients experiencing Long COVID exhibited changes to blood serum proteins indicating activation of the immune system’s complement cascade, altered coagulation, and tissue injury (see the Perspective by Ruf). At the cellular level, Long Covid was linked to aggregates comprising monocytes and platelets. These findings provide a resource of potential biomarkers for diagnosis and may inform directions for treatments. —Sarah H. Ross
RESULTS
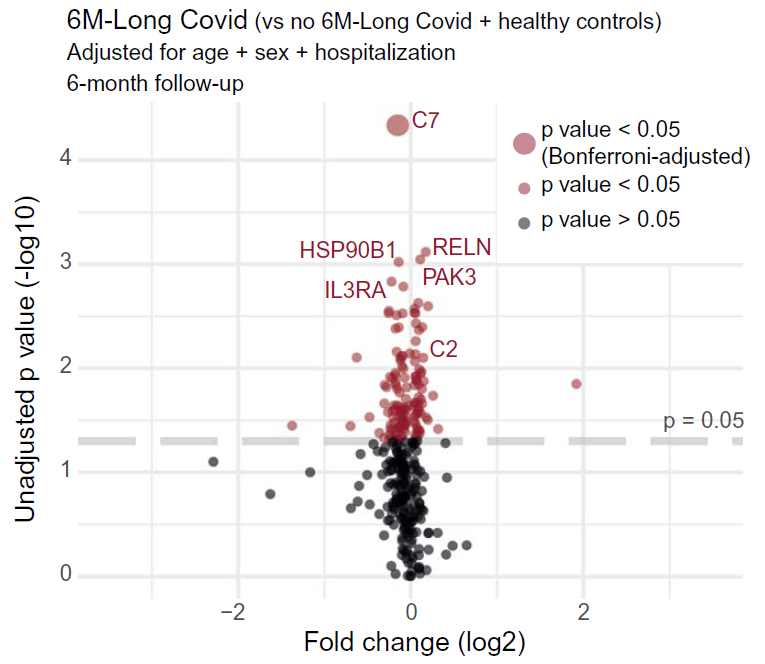
Long Covid patients exhibited increased complement activation during acute disease, which also persisted at 6-month follow-up. The complement system is part of the innate immune system and contributes to immunity and homeostasis by targeting pathogens and damaged cells, among other functions. Interestingly, blood complement levels normalized in Long Covid patients recovering before their 6-month follow-up. The complement system can be activated by various triggers, resulting in formation of the terminal complement complex (TCC), made of the complement components C5b-9. These complexes can integrate into cell membranes and induce cell activation or lysis. Long Covid patients showed imbalanced TCC formation, marked by increased soluble C5bC6 complexes and decreased levels of C7-containing TCC formations that can incorporate into cell membranes. This suggested increased membrane insertion of TCCs in Long Covid patients, contributing to tissue damage. Accordingly, Long Covid patients showed elevated tissue injury markers in blood and a thromboinflammatory signature, characterized by markers of endothelial activation, such as von Willebrand factor (vWF), and red blood cell lysis. Low antithrombin III levels in Long Covid patients were accompanied by signs of increased cleavage by thrombin, a driver of TCC formation. Furthermore, Long Covid patients had elevated platelet activation markers and monocyte–platelet aggregates at 6-month follow-up, particularly in cases where Long Covid persisted for 12 months or more. These patients also showed signs of antibody-mediated activation of the classical complement pathway, which was associated with increased anti-CMV (cytomegalovirus, also known as human herpesvirus 5) and anti-EBV (Epstein-Barr virus) immunoglobulin G (IgG) antibody levels.
CONCLUSION
Our data suggest that active Long Covid is accompanied by a blood protein signature marked by increased complement activation and thromboinflammation, including activated platelets and markers of red blood cell lysis. Tissue injury may also be complement-mediated and, in turn, activate the complement system. Moreover, complement activation may be driven by antigen–antibody complexes, involving autoantibodies and antibodies against herpesviruses, as well as cross-talk with a dysregulated coagulation system. In addition to offering a basis for new diagnostic solutions, our work provides support for clinical research on complement modulators for patients suffering from Long Covid.
Open access: https://www.science.org/doi/10.1126/science.adg7942
Editor’s summary
Some individuals can endure persistent, debilitating symptoms for many months after an initial severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. However, the factors underpinning these health issues, called Long Covid, are poorly understood. Comparing the blood of patients with confirmed SARS-CoV-2 infection with that of uninfected controls, Cervia-Hasler et al. found that patients experiencing Long COVID exhibited changes to blood serum proteins indicating activation of the immune system’s complement cascade, altered coagulation, and tissue injury (see the Perspective by Ruf). At the cellular level, Long Covid was linked to aggregates comprising monocytes and platelets. These findings provide a resource of potential biomarkers for diagnosis and may inform directions for treatments. —Sarah H. Ross
RESULTS
Long Covid patients exhibited increased complement activation during acute disease, which also persisted at 6-month follow-up. The complement system is part of the innate immune system and contributes to immunity and homeostasis by targeting pathogens and damaged cells, among other functions. Interestingly, blood complement levels normalized in Long Covid patients recovering before their 6-month follow-up. The complement system can be activated by various triggers, resulting in formation of the terminal complement complex (TCC), made of the complement components C5b-9. These complexes can integrate into cell membranes and induce cell activation or lysis. Long Covid patients showed imbalanced TCC formation, marked by increased soluble C5bC6 complexes and decreased levels of C7-containing TCC formations that can incorporate into cell membranes. This suggested increased membrane insertion of TCCs in Long Covid patients, contributing to tissue damage. Accordingly, Long Covid patients showed elevated tissue injury markers in blood and a thromboinflammatory signature, characterized by markers of endothelial activation, such as von Willebrand factor (vWF), and red blood cell lysis. Low antithrombin III levels in Long Covid patients were accompanied by signs of increased cleavage by thrombin, a driver of TCC formation. Furthermore, Long Covid patients had elevated platelet activation markers and monocyte–platelet aggregates at 6-month follow-up, particularly in cases where Long Covid persisted for 12 months or more. These patients also showed signs of antibody-mediated activation of the classical complement pathway, which was associated with increased anti-CMV (cytomegalovirus, also known as human herpesvirus 5) and anti-EBV (Epstein-Barr virus) immunoglobulin G (IgG) antibody levels.
CONCLUSION
Our data suggest that active Long Covid is accompanied by a blood protein signature marked by increased complement activation and thromboinflammation, including activated platelets and markers of red blood cell lysis. Tissue injury may also be complement-mediated and, in turn, activate the complement system. Moreover, complement activation may be driven by antigen–antibody complexes, involving autoantibodies and antibodies against herpesviruses, as well as cross-talk with a dysregulated coagulation system. In addition to offering a basis for new diagnostic solutions, our work provides support for clinical research on complement modulators for patients suffering from Long Covid.