Dx Revision Watch
Senior Member (Voting Rights)
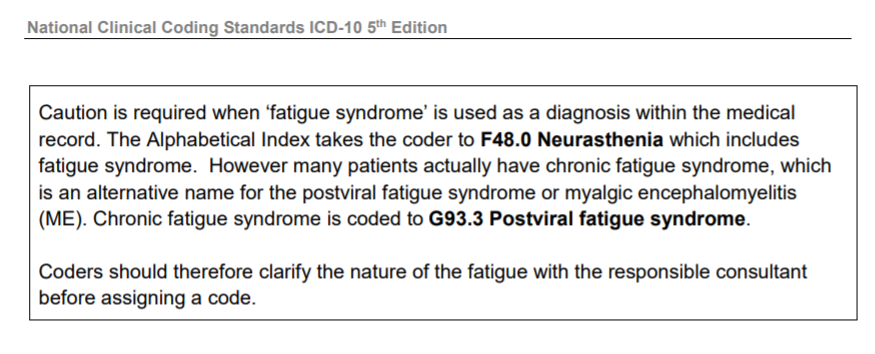
We could do with a stock response ready to send off every time someone confounds ‘ME/CFS’ with ‘chronic fatigue’ something along the lines of:
Is there any thing in the draft NICE guidelines that could be quoted here?
At one point, ICD Revision had got the [US ICD-10-CM] term:
"chronic fatigue, unspecified"
listed under the Synonyms list for ICD-11's 8E49 code. In 2017, I submitted, successfully, for that term to be deleted from the Synonyms list (proposal approved by WHO's Dr Robert Jakob in January 2018, implemented in March 2019).
Last edited: