Immunological profiling in long COVID: overall low grade inflammation and T-lymphocyte senescence and increased monocyte activation correlating with increasing fatigue severity
Background: Many patients with SARS-CoV-2 infection develop long COVID with fatigue as one of the most disabling symptoms. We performed clinical and immune profiling of fatigued and non-fatigued long COVID patients and age- and sex-matched healthy controls (HCs).
Methods: Long COVID symptoms were assessed using patient-reported outcome measures, including the fatigue assessment scale (FAS, scores ≥22 denote fatigue), and followed up to one year after hospital discharge. We assessed inflammation-related genes in circulating monocytes, serum levels of inflammation-regulating cytokines, and leukocyte and lymphocyte subsets, including major monocyte subsets and senescent T-lymphocytes, at 3-6 months post-discharge.
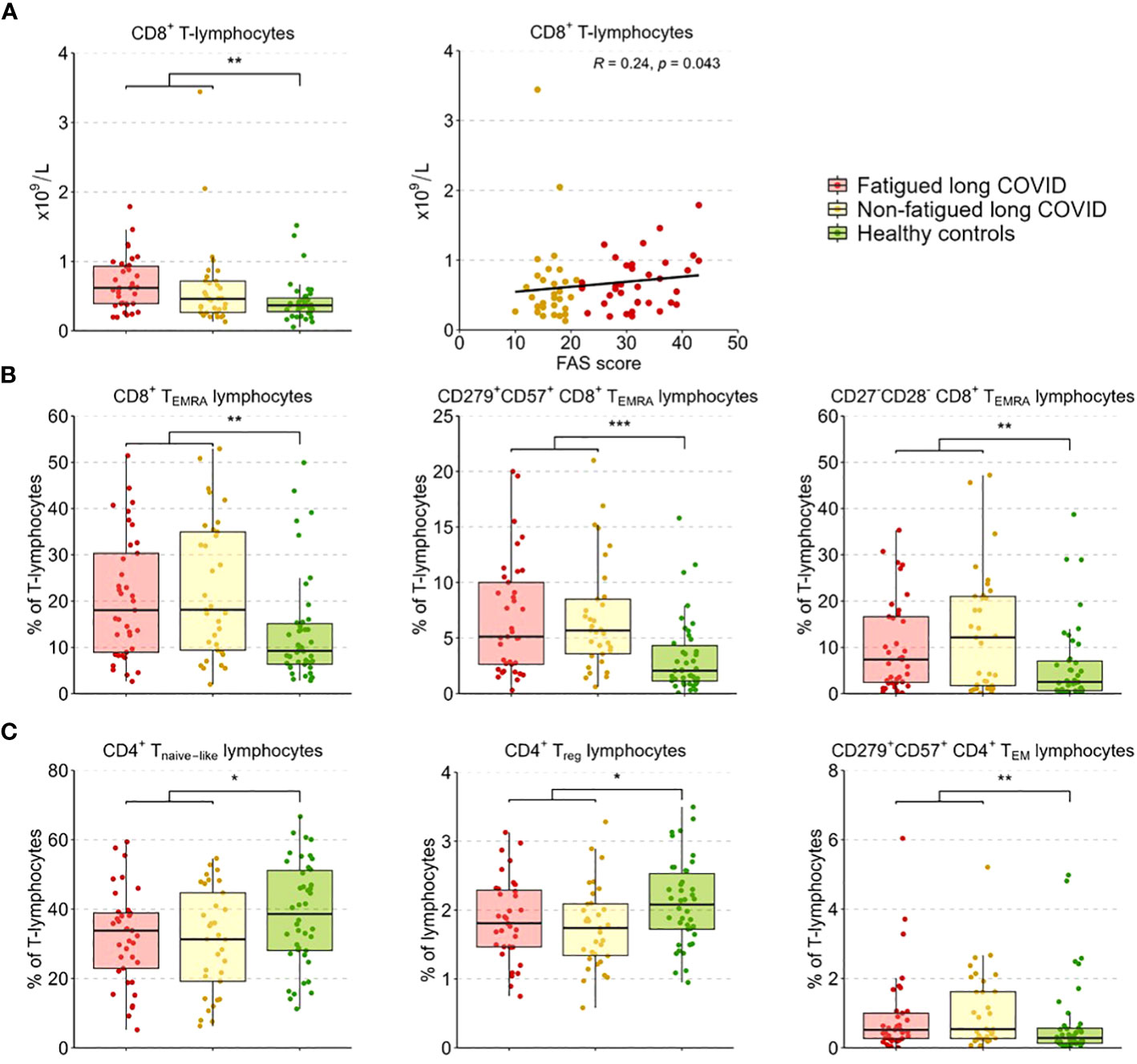
Results: We included 37 fatigued and 36 non-fatigued long COVID patients and 42 HCs. Fatigued long COVID patients represented a more severe clinical profile than non-fatigued patients, with many concurrent symptoms (median 9 [IQR 5.0-10.0] vs 3 [1.0-5.0] symptoms, p<0.001), and signs of cognitive failure (41%) and depression (>24%). Immune abnormalities that were found in the entire group of long COVID patients were low grade inflammation (increased inflammatory gene expression in monocytes, increased serum pro-inflammatory cytokines) and signs of T-lymphocyte senescence (increased exhausted CD8+ TEMRA-lymphocytes). Immune profiles did not significantly differ between fatigued and non-fatigued long COVID groups. However, the severity of fatigue (total FAS score) significantly correlated with increases of intermediate and non-classical monocytes, upregulated gene levels of CCL2, CCL7, and SERPINB2 in monocytes, increases in serum Galectin-9, and higher CD8+ T-lymphocyte counts.
Conclusion: Long COVID with fatigue is associated with many concurrent and persistent symptoms lasting up to one year after hospitalization. Increased fatigue severity associated with stronger signs of monocyte activation in long COVID patients and potentially point in the direction of monocyte-endothelial interaction. These abnormalities were present against a background of immune abnormalities common to the entire group of long COVID patients.
https://www.frontiersin.org/articles/10.3389/fimmu.2023.1254899/full
Background: Many patients with SARS-CoV-2 infection develop long COVID with fatigue as one of the most disabling symptoms. We performed clinical and immune profiling of fatigued and non-fatigued long COVID patients and age- and sex-matched healthy controls (HCs).
Methods: Long COVID symptoms were assessed using patient-reported outcome measures, including the fatigue assessment scale (FAS, scores ≥22 denote fatigue), and followed up to one year after hospital discharge. We assessed inflammation-related genes in circulating monocytes, serum levels of inflammation-regulating cytokines, and leukocyte and lymphocyte subsets, including major monocyte subsets and senescent T-lymphocytes, at 3-6 months post-discharge.
Results: We included 37 fatigued and 36 non-fatigued long COVID patients and 42 HCs. Fatigued long COVID patients represented a more severe clinical profile than non-fatigued patients, with many concurrent symptoms (median 9 [IQR 5.0-10.0] vs 3 [1.0-5.0] symptoms, p<0.001), and signs of cognitive failure (41%) and depression (>24%). Immune abnormalities that were found in the entire group of long COVID patients were low grade inflammation (increased inflammatory gene expression in monocytes, increased serum pro-inflammatory cytokines) and signs of T-lymphocyte senescence (increased exhausted CD8+ TEMRA-lymphocytes). Immune profiles did not significantly differ between fatigued and non-fatigued long COVID groups. However, the severity of fatigue (total FAS score) significantly correlated with increases of intermediate and non-classical monocytes, upregulated gene levels of CCL2, CCL7, and SERPINB2 in monocytes, increases in serum Galectin-9, and higher CD8+ T-lymphocyte counts.
Conclusion: Long COVID with fatigue is associated with many concurrent and persistent symptoms lasting up to one year after hospitalization. Increased fatigue severity associated with stronger signs of monocyte activation in long COVID patients and potentially point in the direction of monocyte-endothelial interaction. These abnormalities were present against a background of immune abnormalities common to the entire group of long COVID patients.
https://www.frontiersin.org/articles/10.3389/fimmu.2023.1254899/full