Andy
Senior Member (Voting rights)
Full title: EBV/HHV-6A dUTPases contribute to Myalgic Encephalomyelitis/Chronic-Fatigue-Syndrome pathophysiology by enhancing TFH cell differentiation and extrafollicular activities
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a chronic, debilitating multisystem illness of unknown etiology for which there is no cure and no diagnostic tests available. Despite increasing evidence implicating EBV and human herpesvirus-6A (HHV-6A) as potential causative infectious agents in a subset of ME/CFS patients, there are few mechanistic studies to address a causal relationship.
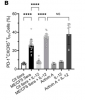
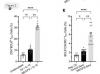
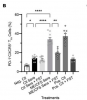
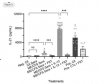
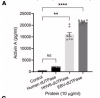
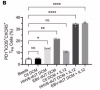
In this study we examined a large ME/CFS cohort (n=351) and 77 controls and demonstrate a significant increase in activin A and IL-21serum levels, which correlated with seropositivity for antibodies to the EBV and HHV-6 protein deoxyuridine-triphosphate nucleotidohydrolase (dUTPase), but not CXCL13. These cytokines are critical for T follicular helper (TFH) cell differentiation, generation of high-affinity antibodies and long-lived plasma cells. Notably, ME/CFS serum was sufficient to drive TFH cell differentiation via an activin A-dependent mechanism. The lack of simultaneous CXCL13 increase with IL-21 indicates impaired TFH-function in ME/CFS. In vitro studies revealed that virus-dUTPases strongly induced activin A secretion while in vivo, EBV-dUTPase induced the formation of splenic marginal zone B and invariant NKTFH cells.
Altogether, our data indicate abnormal germinal center (GC) activity in ME/CFS subjects and highlight a mechanism by which EBV and HHV6-dUTPases may alter GC and extrafollicular Ab responses.
Open access, https://insight.jci.org/articles/view/158193
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a chronic, debilitating multisystem illness of unknown etiology for which there is no cure and no diagnostic tests available. Despite increasing evidence implicating EBV and human herpesvirus-6A (HHV-6A) as potential causative infectious agents in a subset of ME/CFS patients, there are few mechanistic studies to address a causal relationship.
In this study we examined a large ME/CFS cohort (n=351) and 77 controls and demonstrate a significant increase in activin A and IL-21serum levels, which correlated with seropositivity for antibodies to the EBV and HHV-6 protein deoxyuridine-triphosphate nucleotidohydrolase (dUTPase), but not CXCL13. These cytokines are critical for T follicular helper (TFH) cell differentiation, generation of high-affinity antibodies and long-lived plasma cells. Notably, ME/CFS serum was sufficient to drive TFH cell differentiation via an activin A-dependent mechanism. The lack of simultaneous CXCL13 increase with IL-21 indicates impaired TFH-function in ME/CFS. In vitro studies revealed that virus-dUTPases strongly induced activin A secretion while in vivo, EBV-dUTPase induced the formation of splenic marginal zone B and invariant NKTFH cells.
Altogether, our data indicate abnormal germinal center (GC) activity in ME/CFS subjects and highlight a mechanism by which EBV and HHV6-dUTPases may alter GC and extrafollicular Ab responses.
Open access, https://insight.jci.org/articles/view/158193