Andy
Senior Member (Voting rights)
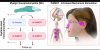
Pre-print, https://www.biorxiv.org/content/10.1101/2020.02.20.958249v1Myalgic encephalomyelitis, ME, previously also known as chronic fatigue syndrome (CFS) is a heterogeneous, debilitating syndrome of unknown etiology responsible for long-lasting disability in millions of patients worldwide. The most well-known symptom of ME is post-exertional malaise, but many patients also experience autonomic dysregulation, cranial nerve dysfunction and signs of immune system activation. Many patients also report a sudden onset of disease following an infection. The brainstem is a suspected focal point in ME pathogenesis and patients with structural impairment to the brainstem often show ME-like symptoms. The brainstem is also where the vagus nerve originates, a critical neuro-immune interface and mediator of the inflammatory reflex which regulate systemic inflammation.
Here we report the results of a randomized, placebo-controlled trial using intranasal mechanical stimulation (INMEST) targeting the vagus nuclei, and higher centers in the brain of ME-patients and induce a sustainable, ~30% reduction in overall symptom scores after eight weeks of treatment. By performing longitudinal, systems-level monitoring of the blood immune system in these patients, we uncover chronic immune activation in ME, as well as immunological correlates of improvement that center around the IL-17 axis, gut-homing immune cells and reduced inflammation. The mechanisms of symptom relief remains to be determined, but transcriptional analyses suggest an upregulation of disease tolerance mechanisms. We wish for these results to bring some hope to patients suffering from ME and inspire researchers to help test our new hypothesis that ME is a condition caused by a failure of inducing disease tolerance upon infection and persistent immune activation.
April 2023 - now published, see here
Achieving symptom relief in patients with ME by targeting the neuro-immune interface and inducing disease tolerance (2020) Rodriguez et al
ETA: Added word, "Pre-print".
Last edited by a moderator: