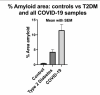
If this turns out to be a solid finding, I wonder how much we'll see it in other diseases as well. The same authors have previously done work that looks at least superficially similar in rheumatoid arthritis. It might be a quite general finding in the end.
Since RA is not generally associated with PEM that suggests these microclots are not causing PEM (at least not alone).
If we ignore that for moment, would it be plausible for PEM to be roughly this sequence of events?
1. A person that was resting begins an activity.
2. The activity also causes a gradually increasing immune activity that favors formation of microclots, but this is initially not noticable due to the physiological changes occurring during activity (blood is being pumped faster and harder, changes in vasodilation, etc).
3. Once the person stops the activity and the cardiovascular system attempts to return to normal, the problems become more noticable. Exhausted cells are now being starved of nutrients and can't recover properly.
4. After some time, the most stressed cells die off and this unusual wave of cells dying triggers alarm signals that result in particular kind of sickness response that looks like PEM. Or maybe merely the signals sent out by these stressed cells is enough to trigger a PEM response which has the purpose of suppressing unnecessary activity.
5. Sleep quality is disrupted, making it even more difficult to properly recover.