2:50:17 Petter Brodin–Sex Hormone Regulation of the Immune System
Main takeaways:
- Peter Brodin's lab is interested in genetics of long COVID, particularly genes involved in antiviral immunity. They hope to report results of a whole genome sequencing study of 110 people with severe long COVID (LC) at the next symposium.
- They are also studying how the immune system is influenced by sex hormones. This may help explain what causes LC, as it is known that females have a higher risk of developing LC.
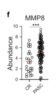
- A study they published this year showed that testosterone therapy causes downregulation of type I interferon genes, which are important for effective immune response against viruses, and upregulation of pro-inflammatory genes, such as TNF and IL-6 [1].
- They found that plamacytoid dendritic cells (pDCs) may be key players in the relationship of sex hormones and immune function as they have high numbers of sex hormone receptors compared to other immune cells.
- Further study of the interaction of sex hormones and immune function may help in understanding the pathophysiology of long COVID.
------------------------------------------------
Peter Brodin's lab is interested in genetics of long COVID. So far, they have done whole genome sequencing on 110 people with well-characterized, severe long COVID which includes objective organ dysfunction. They hypothesize that they will find variants involved in antiviral immunity pathways, such as T cell or NK cell cytotoxicity or type-I interferon. They hope to present results from this at the next PolyBio symposium.
The present talk is mainly about the influence of sex hormone differences on immune responses. He repeats what is known: that infection severity is typically greater in males, but vaccine response and vaccine side effects are more intense, and autoimmune diseases are more frequent in females.
In virtually all countries, before vaccines, COVID killed more males than females. Part of the reason is known to be that a quicker type I interferon (IFN) response helps prevent severe infection, and this type of response is more typically seen in females.

Delayed type-I IFN leads to low T cell response, but a higher B cell response. With delayed type-I IFN, a proinflammatory response driven by TNF and IL-6 may occur, sometimes leading to cytokine storm, severe disease, and/or death.
Several publications have found increased risk of long COVID in females, as well as increased risk in those who are younger.
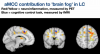
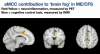
So Peter's lab is interested in how differences in sex chromosomes or sex hormones may be influencing this risk. A study which they designed in 2016 reported their first of several planned reports in September 2024 [1]. They studied the immune systems of people assigned female at birth and taking testosterone therapy in the setting of gender affirming care; this allowed them to separate the influences of sex genes and sex hormones on the immune system. In these individuals, testosterone levels were raised, and estradiol and progesterone were decreased, to levels typical of males.
Using whole blood transcriptomics, they found that genes associated with type-I IFN response were downregulated, while those associated with a pro-inflammatory response (TNF, IL-6, etc) were increased.

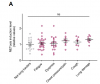
Part of this change could be explained by changes in numbers of cells, for example there were fewer plasmacytoid dendritic cells (pDC) after testosterone therapy, and these are key producers of type-I IFNs.

But cell count does not fully explain lower type-I IFN response because individual pDCs produced less IFN when stimulated by a viral mimic, and this was the most significant change seen in these cells after 3 months of testosterone therapy.

Similarly, monocytes produced less type-I IFN when stimulated by a viral mimic, and they also produced more TNF. The increase in TNF after testosterone therapy was even more pronounced when stimulated with LPS, a chemical produced by bacteria.


pDCs stand out among immune cells as having the highest levels of sex hormone receptors - androgen receptor (AR) for testosterone and ESR receptor for estradiol - in both males and females, as if they are the main sensors of sex hormones, and then modulate the immune system based on hormone levels.

So this study is showing the cross-regulation of IFN-I and TNF. There has previously been evidence of this: when patients are given anti-TNF drugs, their IFN-I response is sometimes increased, which can lead to a lupus-like disease.
He provides some speculation on why sex hormones may cause higher IFN-I and lower TNF in females, and the opposite in males: In females, where reproductive function is a top priority, TNF has been associated with complications in IVF and pregnancy, while IFN-I is an important defense against infections transmitted to a fetus. In contrast, TNF plays an important role in muscle repair and growth, while in the autoimmune condition dermatomyositis, IFN-I is associated with greater muscle wasting, and muscle function may have evolutionarily had a higher priority in males.
In conclusion, Peter says further study of differences in immune function influenced by sex differences may be important for understanding long COVID.
-----
[1]
Immune system adaptation during gender-affirming testosterone treatment, 2024, Lakshmikanth, Brodin et al.