Microvascular Remodeling and Endothelial Dysfunction Across Post-COVID-19 and ME/CFS: Insights from the All Eyes on PCS Study
[Line breaks added]
Background
Post-viral diseases, including post-COVID-19 syndrome (PCS) and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), cause substantial long-term morbidity. Persistent cardiovascular (CV) risk after acute infection highlights the need for accessible tools to quantify microvascular health.
Methods
All Eyes on PCS is a prospective, observational study investigating the retinal microcirculation using retinal vessel analysis (RVA). We compared RVA parameters in 102 PCS patients with 204 age- and sex-matched healthy controls (HC, matched from n = 303).
Secondary matched analyses included never infected controls (NI, n = 96), recovered individuals (n = 102), PCS patients, and ME/CFS patients (n = 62). Laboratory variables, circulating markers of endothelial dysfunction (ED) and inflammation were compared between cohorts and their associations with RVA parameters were examined.
Results
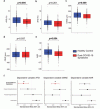
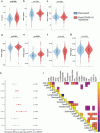
Compared with HC, PCS patients showed reduced venular flicker-induced dilation (3.7 {plus minus} 2.2% vs. 4.5 {plus minus} 2.7%, p = 0.005), narrow retinal arterioles (CRAE, 178.3 {plus minus} 15.5 µm vs. 183.3 {plus minus} 15.9 µm, p = 0.009), and lower arteriolar-to-venular ratio (0.83 {plus minus} 0.06 vs. 0.86 {plus minus} 0.07, p = 0.004).
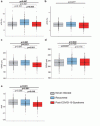
Findings persisted after adjustment for CV factors and remained evident in an extended secondary matched analysis across NI, recovered, and PCS patients. ME/CFS patients showed the most pronounced alterations.
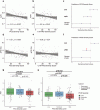
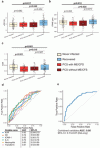
PCS severity correlated with lower AVR (r = -0.21, p = 0.037) and reduced arteriolar FID (r = -0.21, p = 0.039), particularly for neurocognitive symptoms. IL-6, ICAM-1 and VCAM-1 were elevated in PCS and ME/CFS and lower AVR correlated with inflammatory and iron-related markers (all adjusted p < 0.01). A combined model discriminated ME/CFS patients with good accuracy (AUC = 0.80).
Conclusions
PCS is associated with persistent ED, most pronounced in ME/CFS patients and linked to symptom severity and ongoing inflammation. RVA may provide a noninvasive, readout of ED in post-viral syndromes.
Web | DOI | PDF | medRxiv | Preprint
Wallraven, Timon; Günthner, Roman; Lethen, Isabelle; Ribeiro, Andrea; Lech, Maciej; Oertel, Frederike Cosima; Rees, Lukas; Haller, Bernhard; Streese, Lukas; Hanssen, Henner; Wunderle, Michael; Schmaderer, Christoph
[Line breaks added]
Background
Post-viral diseases, including post-COVID-19 syndrome (PCS) and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), cause substantial long-term morbidity. Persistent cardiovascular (CV) risk after acute infection highlights the need for accessible tools to quantify microvascular health.
Methods
All Eyes on PCS is a prospective, observational study investigating the retinal microcirculation using retinal vessel analysis (RVA). We compared RVA parameters in 102 PCS patients with 204 age- and sex-matched healthy controls (HC, matched from n = 303).
Secondary matched analyses included never infected controls (NI, n = 96), recovered individuals (n = 102), PCS patients, and ME/CFS patients (n = 62). Laboratory variables, circulating markers of endothelial dysfunction (ED) and inflammation were compared between cohorts and their associations with RVA parameters were examined.
Results
Compared with HC, PCS patients showed reduced venular flicker-induced dilation (3.7 {plus minus} 2.2% vs. 4.5 {plus minus} 2.7%, p = 0.005), narrow retinal arterioles (CRAE, 178.3 {plus minus} 15.5 µm vs. 183.3 {plus minus} 15.9 µm, p = 0.009), and lower arteriolar-to-venular ratio (0.83 {plus minus} 0.06 vs. 0.86 {plus minus} 0.07, p = 0.004).
Findings persisted after adjustment for CV factors and remained evident in an extended secondary matched analysis across NI, recovered, and PCS patients. ME/CFS patients showed the most pronounced alterations.
PCS severity correlated with lower AVR (r = -0.21, p = 0.037) and reduced arteriolar FID (r = -0.21, p = 0.039), particularly for neurocognitive symptoms. IL-6, ICAM-1 and VCAM-1 were elevated in PCS and ME/CFS and lower AVR correlated with inflammatory and iron-related markers (all adjusted p < 0.01). A combined model discriminated ME/CFS patients with good accuracy (AUC = 0.80).
Conclusions
PCS is associated with persistent ED, most pronounced in ME/CFS patients and linked to symptom severity and ongoing inflammation. RVA may provide a noninvasive, readout of ED in post-viral syndromes.
Web | DOI | PDF | medRxiv | Preprint