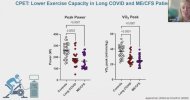
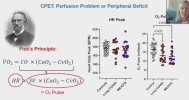
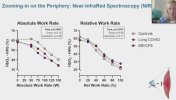
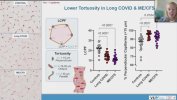
Let me know if we've already seen/discussed this elsewhere...
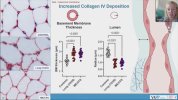
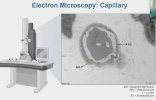
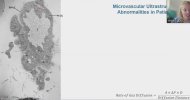
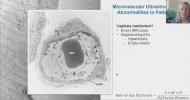
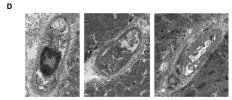
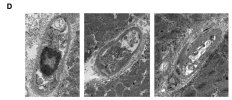
Towards the end of his talk Rob Wüst mentioned that they found the lumen (interior space) of the capillaries was much reduced in the ME/CFS and LC cohorts compared to controls (and I'm not 100% sure but I think these were his 60-days-of-bed-rest controls). It sounds like it was just kind of a surprise observation one of his grad students made, and needs to be confirmed, but there was a total separation between patients and controls so it's a little bit intriguing.
He suggested this thicker capillary wall / reduced interior space could be messing with gas exchange into tissue and could be upstream of problems pwME have with muscles. I'm wondering, if this result turns out to be real in muscle, is it possible that the same thing could be wrong with capillaries in the brain?