I'm copy-pasting a Twitter thread that I wrote about a poster presentation by Slaghekke et al. (Wüst team). I've added an extra sentence here and there (e.g. on Poiseille's law) to explain a bit more.
You can read a general take by ME/CFS Science in the tweet below.
Poster: https://mecfs-research.org/wp-content/uploads/2025/04/Anouk-Slaghekke_Poster_Conference_2025.pdf
Presentation:
 What's interesting about these findings, is that they had moderately strong correlation with CPET performance! That's in addition to there being a clean separation between healthy controls vs. LC* & ME
What's interesting about these findings, is that they had moderately strong correlation with CPET performance! That's in addition to there being a clean separation between healthy controls vs. LC* & ME
I find these findings really exciting!
Some thoughts & caveats
Unroll available on Thread Reader

First, a methodology question
The x-axis appears to be a composite measure of 'CF * COL4 (Lumen)'
(Lumen is the internal space of the capillary, through which the blood flows)
In the caption it's called 'CF*Lumen'
I don't know what CF means. Capillary flow? Collagen fiber?
Also, you can create all kinds of composite measures, so this increases the risk of p-hacking.
But if we leave that aside and assume that there's a benign reason for it, we're left with some fascinating results.. because these findings aren't unique to LC/ME
Scleromyositis is a condition where there's significant thickening of the basement membrane, as well as high amounts (~two-thirds) of BM 'reduplication' (layers)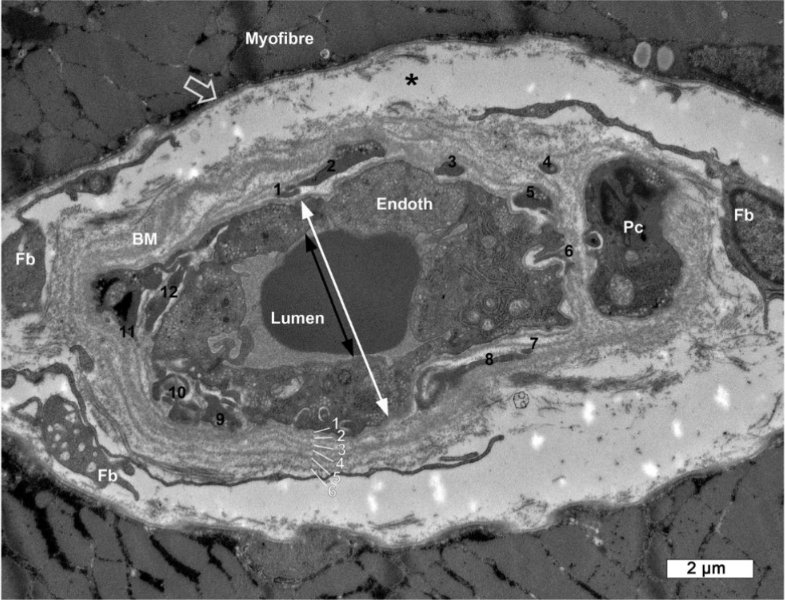
Image of a capillary in Scleromyositis. You can see the 6 layers of the basement membrane (should normally be just one)
Now, Slaghekke doesn't note the frequency of reduplication in LC/ME samples, and I can't find comparable BM measurements in scleromyositis.
But we can assume it's a worse amount of BM thickening, as well as that it's systemic (including the lungs).
Image of reduplication in ME/CFS, from the poster
We don't yet know if it's systemic in LC/ME or only in muscle capillaries.
Regarding the lumen diameter, I can't easily find data for Scleromyositis, so it's hard to compare. (This matters, as I discuss later)
Now the interesting question: how do people with scleromyositis react to exercise? Do they get PEM?
They do have a 'disproportional response to exercise', getting easily fatigued. This is not just due to capillary BM thickening in muscles, but also in the lungs and at the heart.
However, scleromyositis patients do improve with exercise over time! No PEM. This suggests ME/CFS has more going on.
Still, it has an effect on 1-day CPET performance. I asked Claude (AI) to calculate how much these variables would affect oxygen supply, and was pretty shocked!
The diffusion would be reduced by 25-35% (thicker layer to cross)

Flow however is much more severely impacted by reduced diameter (see the r⁴ term). A 29% reduced diameter leads to a 75% reduced blood flow through the capillaries! (Reduced volume at reduced speeds) I'm not familiar with this, maybe this is compensated somewhat by higher pressure?
Together, these would lead to 81-84% reduction in capacity!
(I don't know how this compares to scleromyositis, because of the unknown lumen space. I'm on completely unfamiliar terrain, but I think lumen space might be significantly wider and more variable in Scleromyositis. This would imply less flow issues and more oxygen delivery)
Now, 81-84% doesn't quite match the observed reductions in power output, which looks more like 30-60%.
This could be due to compensatory mechanisms, such as anaerobic respiration.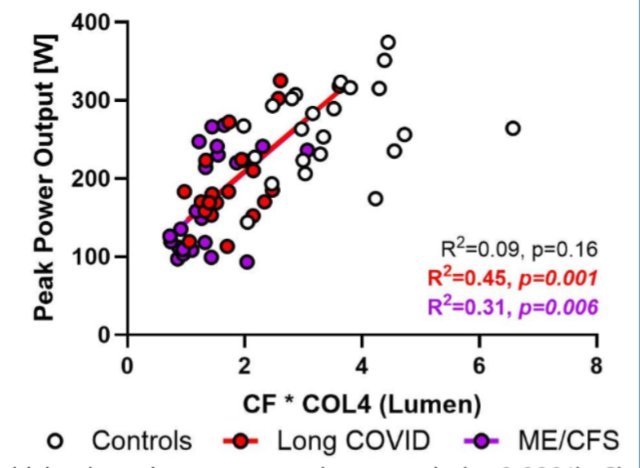
Nonetheless, it would be great if we can resolve this, especially by increasing the lumen space, which would have the largest effect on capacity
1 drug that would normally help to widen capillaries is Fasudil, which works by relaxing the pericytes attached to endothelial cells..
Well, I happen to know of 10+ people who took Fasudil. And iirc, no response.
How could that be? I don't know. Perhaps the basement membrane is stiff enough to keep the capillaries' lumen compressed regardless of pericyte relaxation?
This all leaves me with a bunch of questions, like all good research does. I hope we see a replication attempt soon, as well as highly needed follow-up research. I'm excited!
- What would happen if we let people do a CPET in a high-oxygen setting? HBOT could give up to 2x the oxygen (2.4ATA, my calculations), which wouldn't be enough to compensate for 5x capacity loss, but might still be interesting?
You can read a general take by ME/CFS Science in the tweet below.
Poster: https://mecfs-research.org/wp-content/uploads/2025/04/Anouk-Slaghekke_Poster_Conference_2025.pdf
Presentation:
 What's interesting about these findings, is that they had moderately strong correlation with CPET performance! That's in addition to there being a clean separation between healthy controls vs. LC* & ME
What's interesting about these findings, is that they had moderately strong correlation with CPET performance! That's in addition to there being a clean separation between healthy controls vs. LC* & MEI find these findings really exciting!
Some thoughts & caveats
Unroll available on Thread Reader

First, a methodology question
The x-axis appears to be a composite measure of 'CF * COL4 (Lumen)'
(Lumen is the internal space of the capillary, through which the blood flows)
In the caption it's called 'CF*Lumen'
I don't know what CF means. Capillary flow? Collagen fiber?

Also, you can create all kinds of composite measures, so this increases the risk of p-hacking.
But if we leave that aside and assume that there's a benign reason for it, we're left with some fascinating results.. because these findings aren't unique to LC/ME
Scleromyositis is a condition where there's significant thickening of the basement membrane, as well as high amounts (~two-thirds) of BM 'reduplication' (layers)

Image of a capillary in Scleromyositis. You can see the 6 layers of the basement membrane (should normally be just one)
Now, Slaghekke doesn't note the frequency of reduplication in LC/ME samples, and I can't find comparable BM measurements in scleromyositis.
But we can assume it's a worse amount of BM thickening, as well as that it's systemic (including the lungs).

Image of reduplication in ME/CFS, from the poster
We don't yet know if it's systemic in LC/ME or only in muscle capillaries.
Regarding the lumen diameter, I can't easily find data for Scleromyositis, so it's hard to compare. (This matters, as I discuss later)
Now the interesting question: how do people with scleromyositis react to exercise? Do they get PEM?
They do have a 'disproportional response to exercise', getting easily fatigued. This is not just due to capillary BM thickening in muscles, but also in the lungs and at the heart.
However, scleromyositis patients do improve with exercise over time! No PEM. This suggests ME/CFS has more going on.
Still, it has an effect on 1-day CPET performance. I asked Claude (AI) to calculate how much these variables would affect oxygen supply, and was pretty shocked!
The diffusion would be reduced by 25-35% (thicker layer to cross)


Flow however is much more severely impacted by reduced diameter (see the r⁴ term). A 29% reduced diameter leads to a 75% reduced blood flow through the capillaries! (Reduced volume at reduced speeds) I'm not familiar with this, maybe this is compensated somewhat by higher pressure?
Together, these would lead to 81-84% reduction in capacity!

(I don't know how this compares to scleromyositis, because of the unknown lumen space. I'm on completely unfamiliar terrain, but I think lumen space might be significantly wider and more variable in Scleromyositis. This would imply less flow issues and more oxygen delivery)
Now, 81-84% doesn't quite match the observed reductions in power output, which looks more like 30-60%.
This could be due to compensatory mechanisms, such as anaerobic respiration.

Nonetheless, it would be great if we can resolve this, especially by increasing the lumen space, which would have the largest effect on capacity
1 drug that would normally help to widen capillaries is Fasudil, which works by relaxing the pericytes attached to endothelial cells..
Well, I happen to know of 10+ people who took Fasudil. And iirc, no response.
How could that be? I don't know. Perhaps the basement membrane is stiff enough to keep the capillaries' lumen compressed regardless of pericyte relaxation?
This all leaves me with a bunch of questions, like all good research does. I hope we see a replication attempt soon, as well as highly needed follow-up research. I'm excited!

- What would happen if we let people do a CPET in a high-oxygen setting? HBOT could give up to 2x the oxygen (2.4ATA, my calculations), which wouldn't be enough to compensate for 5x capacity loss, but might still be interesting?
Last edited by a moderator:

