Tissue Weakness and Fragility increases with exposure along with Mitochondrial Fragmentation
Figures 6A-C
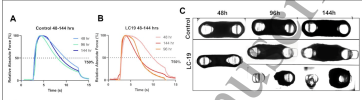
A) Relative absolute force at 50 Hz for Control. The dotted line indicates the time taken for the force to drop to 50% of its peak value under sustained tetanic stimulation of 50 Hz (T50%)
B) Relative absolute force at 50 Hz for LC-19 tissues over time.
C) Brightfield images of progressive muscle exposure to Control and LC sera
In A, the blue lines are the control at the different serum exposure times. B, with the orange lines, is the same for the LC serum. The lines are the force exerted by the tissue when stimulated. Note that the charts are of relative force, for that tissue and that exposure. So, all the experiments achieve 100% of the force at the peak, just because that is how the chart is set up. But, tissue with longer exposure to the LC serum cannot sustain the force as long.
Figure 6c shows examples of the tissue, with the LC tissue looking less robust over time.
If you look at Figure 6f, I'm not sure that there are really differences in mitochondrial branching between groups. The differences between groups don't seem to be significant. The very small samples sizes don't help.
There are some photos of the cells with the mitochondria looking more odd in the LC and ME/CFS samples. This is a bit shaky, and the authors seem to acknowledge that, noting the very small sample sizes. It does look though, that the muscle tissue is less able to handle the stress of being incubated with LC or ME/CFS serum rather than healthy serum.
That's the last of the results.
So, to test the tissue strength, they used some of the engineered muscle tissue and applied LC and control sera for the three different lengths of time. Note, no ME/CFS sera in this experiment.For this experiment, we used tissues obtained from the same batch of encapsulation to avoid variability in handling and exposed them to LC-19 and control sera for 48, 96 and 144 hours.
I think this is interesting. They weren't testing tear strength of the tissue here, they were testing the ability of the muscle to contract (as they did before).The diseased tissues were weaker as evidenced by a lower T50% compared to the controls (Figure 6a)
Figures 6A-C

A) Relative absolute force at 50 Hz for Control. The dotted line indicates the time taken for the force to drop to 50% of its peak value under sustained tetanic stimulation of 50 Hz (T50%)
B) Relative absolute force at 50 Hz for LC-19 tissues over time.
C) Brightfield images of progressive muscle exposure to Control and LC sera
In A, the blue lines are the control at the different serum exposure times. B, with the orange lines, is the same for the LC serum. The lines are the force exerted by the tissue when stimulated. Note that the charts are of relative force, for that tissue and that exposure. So, all the experiments achieve 100% of the force at the peak, just because that is how the chart is set up. But, tissue with longer exposure to the LC serum cannot sustain the force as long.
Figure 6c shows examples of the tissue, with the LC tissue looking less robust over time.
Tissue survival decreased sharply with time for both diseased and control groups, but the decline was two-fold higher for the former than the latter.
If you look at Figure 6f, I'm not sure that there are really differences in mitochondrial branching between groups. The differences between groups don't seem to be significant. The very small samples sizes don't help.
We then analyzed mitochondrial morphology and quantified mitochondrial networks for each time point. Our data indicated a decline in mitochondrial branching and mean branch length. The mitochondria not only assumed the familiar globular geometry observed during fragmentation but also toroidal conformations indicating changes in mitochondrial membrane potential at 144 hours of patient serum exposure (Figure 6F, G).
The toroidal conformations are typically observed with FCCP administration at high dosages due to depolarization of the mitochondrial membrane, resulting in a drop in mitochondrial membrane potential and uncoupling of mitochondrial oxidative phosphorylation and ATP synthesis (47). These findings confirmed mitochondrial stress induced by systemic stress factors coupled with myotube atrophy at longer exposures. Our results of prolonged exposure to patient sera further warrant investigation due to small sample size but signpost at progressive deterioration of muscle structure, function and mitochondrial energy production, mimicking the conditions observed in patients.
There are some photos of the cells with the mitochondria looking more odd in the LC and ME/CFS samples. This is a bit shaky, and the authors seem to acknowledge that, noting the very small sample sizes. It does look though, that the muscle tissue is less able to handle the stress of being incubated with LC or ME/CFS serum rather than healthy serum.
That's the last of the results.
Last edited:



