Mechanisms of sex differences in acute and long COVID sequelae in mice
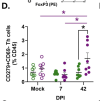
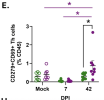
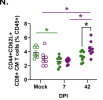
While males are more likely to suffer severe outcomes during acute COVID-19, a greater proportion of females develop post-acute sequalae of COVID-19 (PASC) despite similar rates of infection. To identify mechanisms of PASC, mice were infected with SARS-CoV-2 and viral, inflammatory, and behavioral outcomes were evaluated through 84 days post infection. Sex differences were not observed in virus replication or persistence of viral RNA in pulmonary or extrapulmonary tissues in acute or PASC phases. Following recovery from infection, female mice exhibited persistent neurocognitive and behavioral impairments, along with greater frequencies of inflammatory myeloid subsets, neuroinflammation, and dysregulated T cell subsets, including Tregs. Sex differences in inflammation and cognitive phenotypes during PASC were mediated by the presence of two X chromosomes. XX animals independent of chromosome Y presented with neuroinflammation and PASC along with infection-induced upregulation of the X-linked genes Xist and Tlr7 that regulate inflammation and chronic disease outcomes.
Link | PDF | Preprint: BioRxiv | Open Access
Jennifer A Liu; Sabal Chaulagain; Patrick S Creisher; Weizhi Zhong; Tianle Zhang; Maraake Taddese; Karly Shi; Han-Sol Park; Haley Hcnir; Arthur P Arnold; Ralph S Baric; Natasha B Barahona; Elizabeth B Engler-Chiurazzi; Kevin J Zwezdaryk; Chloe L Thio; Ashwin Balagopal; Jack R Harkema; Elizabeth A Thompson; Andrew Pekosz; Andrea L Cox; Sabra L Klein
While males are more likely to suffer severe outcomes during acute COVID-19, a greater proportion of females develop post-acute sequalae of COVID-19 (PASC) despite similar rates of infection. To identify mechanisms of PASC, mice were infected with SARS-CoV-2 and viral, inflammatory, and behavioral outcomes were evaluated through 84 days post infection. Sex differences were not observed in virus replication or persistence of viral RNA in pulmonary or extrapulmonary tissues in acute or PASC phases. Following recovery from infection, female mice exhibited persistent neurocognitive and behavioral impairments, along with greater frequencies of inflammatory myeloid subsets, neuroinflammation, and dysregulated T cell subsets, including Tregs. Sex differences in inflammation and cognitive phenotypes during PASC were mediated by the presence of two X chromosomes. XX animals independent of chromosome Y presented with neuroinflammation and PASC along with infection-induced upregulation of the X-linked genes Xist and Tlr7 that regulate inflammation and chronic disease outcomes.
Link | PDF | Preprint: BioRxiv | Open Access