jnmaciuch
Senior Member (Voting Rights)
IFNγ causes mitochondrial dysfunction and oxidative stress in myositis
Catalina Abad, Iago Pinal-Fernandez, Clement Guillou, Gwladys Bourdenet, Laurent Drouot, Pascal Cosette, Margherita Giannini, Lea Debrut, Laetitia Jean, Sophie Bernard, Damien Genty, Rachid Zoubairi, Isabelle Remy-Jouet, Bernard Geny, Christian Boitard, Andrew Mammen, Alain Meyer & Olivier BoyerAbstract
[Paragraph breaks added]
Idiopathic inflammatory myopathies (IIMs) are severe autoimmune diseases with poorly understood pathogenesis and unmet medical needs. Here, we examine the role of interferon γ (IFNγ) using NOD female mice deficient in the inducible T cell co-stimulator (Icos), which have previously been shown to develop spontaneous IFNγ-driven myositis mimicking human disease.
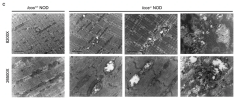
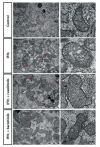
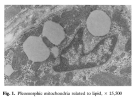
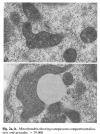
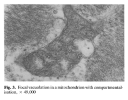
Using muscle proteomic and spatial transcriptomic analyses we reveal profound myofiber metabolic dysregulation in these mice. In addition, we report muscle mitochondrial abnormalities and oxidative stress in diseased mice. Supporting a pathogenic role for oxidative stress, treatment with a reactive oxygen species (ROS) buffer compound alleviated myositis, preserved muscle mitochondrial ultrastructure and respiration, and reduced inflammation. Mitochondrial anomalies and oxidative stress were diminished following anti-IFNγ treatment.
Further transcriptomic analysis in IIMs patients and human myoblast in vitro studies supported the link between IFNγ and mitochondrial dysfunction observed in mice. These results suggest that mitochondrial dysfunction, ROS and inflammation are interconnected in a self-maintenance loop, opening perspectives for mitochondria therapy and/or ROS targeting drugs in myositis.
Link (open access)