It's nearly two years since this was preprinted.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Genome-wide Association Study of Long COVID, 2023, Lammi et al.
- Thread starter chillier
- Start date
-
- Tags
- foxp4 gwas long covid
Nightsong
Senior Member (Voting Rights)
Now published in Nature Genetics:
https://www.nature.com/articles/s41588-025-02100-w
https://www.nature.com/articles/s41588-025-02100-w
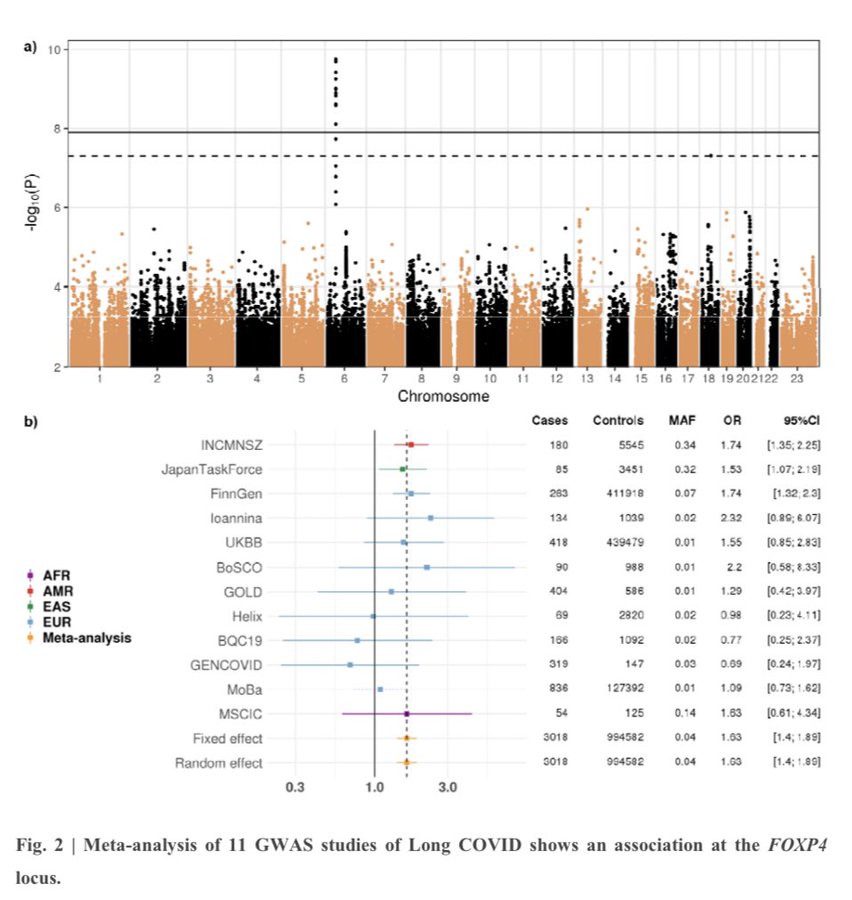
Abstract said:Infections can lead to persistent symptoms and diseases such as shingles after varicella zoster or rheumatic fever after streptococcal infections. Similarly, severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) infection can result in long coronavirus disease (COVID), typically manifesting as fatigue, pulmonary symptoms and cognitive dysfunction. The biological mechanisms behind long COVID remain unclear. We performed a genome-wide association study for long COVID including up to 6,450 long COVID cases and 1,093,995 population controls from 24 studies across 16 countries. We discovered an association of FOXP4 with long COVID, independent of its previously identified association with severe COVID-19. The signal was replicated in 9,500 long COVID cases and 798,835 population controls. Given the transcription factor FOXP4’s role in lung physiology and pathology, our findings highlight the importance of lung function in the pathophysiology of long COVID.
Andy
Senior Member (Voting rights)
"Phenotype definitions
We used the following criteria for assigning case–control status for long COVID aligning with the World Health Organization guidelines1 (Supplementary Note; https://github.com/long-covid-hg/LongCovidTools/blob/main/PhenotypeDefinitions_LongCOVID_v1.docx). Study participants were defined as long COVID cases if, at least three months since SARS-CoV-2 infection or COVID-19 onset, they met any of the following criteria:
We used two long COVID case definitions, a strict definition requiring a test-verified SARS-CoV-2 infection and a broad definition including self-reported or clinician-diagnosed SARS-CoV-2 infection (any long COVID)."
We used the following criteria for assigning case–control status for long COVID aligning with the World Health Organization guidelines1 (Supplementary Note; https://github.com/long-covid-hg/LongCovidTools/blob/main/PhenotypeDefinitions_LongCOVID_v1.docx). Study participants were defined as long COVID cases if, at least three months since SARS-CoV-2 infection or COVID-19 onset, they met any of the following criteria:
- Presence of one or more self-reported COVID-19 symptoms that cannot be explained by an alternative diagnosis
- Report of ongoing substantial impact on day-to-day activities
- Any diagnosis codes of long COVID (for example, post-COVID-19 condition, ICD-10 code U09(.9))
We used two long COVID case definitions, a strict definition requiring a test-verified SARS-CoV-2 infection and a broad definition including self-reported or clinician-diagnosed SARS-CoV-2 infection (any long COVID)."
Jonathan Edwards
Senior Member (Voting Rights)
I'm wondering if I've misunderstood it but is it weird that it turned up so few SNPs? It seems like a biggish study (in terms of the number of controls, at any rate, but maybe that's not what counts).
Maybe it is a sign that if you have too dilute or non-specific a disease cohort you get nothing much. That would be helpful to confirm. It might partly explain why the first study of the Leeds biobank cohort produced little, despite having 2000 cases.
Dolphin
Senior Member (Voting Rights)
https://www.eurekalert.org/news-releases/1084924
News Release 22-May-2025
FOXP4 gene identified in the first large-scale genetic study on Long COVID with participation of the GCAT
Peer-Reviewed Publication
Germans Trias i Pujol Research Institute
GCAT is the only Spanish project to contribute to this international study
New insights into the complexities of Long COVID have been brought to light, thanks to an international collaborative effort with the participation of researchers from GCATGenomes for life, a strategic project of the Germans Trias i Pujol Research Institute (IGTP). A study published yesterday in Nature Genetics used the vast data from the COVID-19 Host Genetics Initiative to perform the first study examining the entire genetic code specifically focused on Long COVID.
The term "Long COVID", officially recognised by the World Health Organisation, describes symptoms that persist for months after the acute phase of a COVID-19 infection has subsided. This condition has become a significant concern worldwide with symptoms ranging from pulmonary issues and fatigue to cognitive disturbances.
A study published in the journal Nature Genetics has identified the first genome-wide significant association for Long COVID, which has been found at the FOXP4 gene. The study, conducted under the COVID-19 Host Genetics Initiative, included up to 6,450 individuals diagnosed with Long COVID and over a million controls from 16 countries. The analysis was conducted using data from participants in the GCAT cohort, coordinated by IGTP, that have been monitoring the COVID-19 pandemic in the Catalan population since 2020 with the collaboration of the Barcelona Institute for Global Health (ISGlobal)-a centre supported by the "la Caixa" Foundation, the COVICAT cohort (Cohort COVID in Catalonia).
The results suggest that the link between the FOXP4 gene and Long COVID is deeply connected to both how our lungs work and how our immune system responds to infections. Additionally, the researchers have discovered a strong relationship between severe cases of COVID-19 and Long COVID, in line with previous research. This raises the question: do all genetic variants that make someone more susceptible to COVID-19 and more affected by it also increase the risk of Long COVID? Not necessarily. The study has found that, while most genetic variants primarily affect how we respond to the SARS-CoV-2 virus or the severity of COVID-19, those related to the FOXP4 gene seem to play a significant role in the onset of Long COVID. This highlights potential biological factors that are crucial in Long COVID, associated with lung function and our immune response.
Rafael De Cid, scientific director of the GCAT project at IGTP and author of the study, states: "The study's findings will help categorise risk factors for Long COVID, a complex condition shaped by both risk factors-such as disease severity, chronic conditions, and obesity-and protective factors like vaccination, sleep, and exercise. These opposing influences contribute to symptom persistence and subtype variability, including gender differences. Their consideration and follow-up in future studies will aid in distinguishing subtypes and advancing personalised prevention and treatment strategies."
Long COVID continues to be a multifaceted disease with a wide variety of symptoms. Further research is needed to fully understand the mechanisms behind it. Nonetheless, this study provides genetic evidence of the pivotal role that lung health and function might have in the progression of this post-COVID condition.
Journal
Nature Genetics
DOI
10.1038/s41588-025-02100-w
Method of Research
Meta-analysis
Subject of Research
People
Article Title
Genome-wide association study of long COVID
Article Publication Date
21-May-2025
News Release 22-May-2025
FOXP4 gene identified in the first large-scale genetic study on Long COVID with participation of the GCAT
Peer-Reviewed Publication
Germans Trias i Pujol Research Institute
GCAT is the only Spanish project to contribute to this international study
New insights into the complexities of Long COVID have been brought to light, thanks to an international collaborative effort with the participation of researchers from GCATGenomes for life, a strategic project of the Germans Trias i Pujol Research Institute (IGTP). A study published yesterday in Nature Genetics used the vast data from the COVID-19 Host Genetics Initiative to perform the first study examining the entire genetic code specifically focused on Long COVID.
The term "Long COVID", officially recognised by the World Health Organisation, describes symptoms that persist for months after the acute phase of a COVID-19 infection has subsided. This condition has become a significant concern worldwide with symptoms ranging from pulmonary issues and fatigue to cognitive disturbances.
A study published in the journal Nature Genetics has identified the first genome-wide significant association for Long COVID, which has been found at the FOXP4 gene. The study, conducted under the COVID-19 Host Genetics Initiative, included up to 6,450 individuals diagnosed with Long COVID and over a million controls from 16 countries. The analysis was conducted using data from participants in the GCAT cohort, coordinated by IGTP, that have been monitoring the COVID-19 pandemic in the Catalan population since 2020 with the collaboration of the Barcelona Institute for Global Health (ISGlobal)-a centre supported by the "la Caixa" Foundation, the COVICAT cohort (Cohort COVID in Catalonia).
The results suggest that the link between the FOXP4 gene and Long COVID is deeply connected to both how our lungs work and how our immune system responds to infections. Additionally, the researchers have discovered a strong relationship between severe cases of COVID-19 and Long COVID, in line with previous research. This raises the question: do all genetic variants that make someone more susceptible to COVID-19 and more affected by it also increase the risk of Long COVID? Not necessarily. The study has found that, while most genetic variants primarily affect how we respond to the SARS-CoV-2 virus or the severity of COVID-19, those related to the FOXP4 gene seem to play a significant role in the onset of Long COVID. This highlights potential biological factors that are crucial in Long COVID, associated with lung function and our immune response.
Rafael De Cid, scientific director of the GCAT project at IGTP and author of the study, states: "The study's findings will help categorise risk factors for Long COVID, a complex condition shaped by both risk factors-such as disease severity, chronic conditions, and obesity-and protective factors like vaccination, sleep, and exercise. These opposing influences contribute to symptom persistence and subtype variability, including gender differences. Their consideration and follow-up in future studies will aid in distinguishing subtypes and advancing personalised prevention and treatment strategies."
Long COVID continues to be a multifaceted disease with a wide variety of symptoms. Further research is needed to fully understand the mechanisms behind it. Nonetheless, this study provides genetic evidence of the pivotal role that lung health and function might have in the progression of this post-COVID condition.
Journal
Nature Genetics
DOI
10.1038/s41588-025-02100-w
Method of Research
Meta-analysis
Subject of Research
People
Article Title
Genome-wide association study of long COVID
Article Publication Date
21-May-2025
Dolphin
Senior Member (Voting Rights)
https://www.eurekalert.org/news-releases/1084764
News Release 21-May-2025
A gene variant increases the risk of long COVID
Peer-Reviewed Publication
Karolinska Institutet
An international team of researchers has found a genetic link to long-term symptoms after COVID-19. The identified gene variant is located close to the FOXP4 gene, which is known to affect lung function. The study, published in Nature Genetics, was led by researchers at Karolinska Institutet in Sweden and the Institute for Molecular Medicine Finland.
Biological causes behind persistent symptoms after COVID-19 infection, known as long COVID or post-COVID, remain unclear. Common symptoms include fatigue, cognitive difficulties, and breathing problems, which can reduce quality of life.
In an international collaboration – the Long COVID Host Genetics Initiative – researchers have analysed genetic data from 6,450 long COVID patients and more than a million controls across 24 studies from 16 countries. They found a gene variant that increases the risk of long COVID by about 60 percent. The genetic association was confirmed in an independent analysis involving an additional 9,500 cases.
Impaired lung function plays a key role
The gene variant is located right next to the gene FOXP4, which is involved in lung development and lung disease.
“Our findings suggest that impaired lung function plays a key role in developing long COVID,” says Hugo Zeberg, senior lecturer at the Department of Physiology and Pharmacology, Karolinska Institutet, and one of the lead researchers of the study. “While this gene variant significantly increases risk, it’s important to recognise it as just one part of a much larger puzzle,” he continues.
“Genetic studies can provide insights into disease risk factors and are particularly powerful for diseases where the exact mechanisms remain unknown,” says Hanna Ollila, FIMM-EMBL group leader at the Institute for Molecular Medicine Finland, University of Helsinki, and researcher at the Department of Anesthesia and Center for Genomic Medicine at Massachusetts General Hospital, who co-led the study.
See the published paper for information about funding and potential conflicts of interest.
Publication: “Genome-wide association study of long COVID”, Vilma Lammi et al., Nature Genetics, online 21 May 2025, doi: 10.1038/s41588-025-02100-w.
Journal
Nature Genetics
DOI
10.1038/s41588-025-02100-w
Subject of Research
People
Article Title
Genome-wide association study of long COVID
Article Publication Date
21-May-2025
News Release 21-May-2025
A gene variant increases the risk of long COVID
Peer-Reviewed Publication
Karolinska Institutet
An international team of researchers has found a genetic link to long-term symptoms after COVID-19. The identified gene variant is located close to the FOXP4 gene, which is known to affect lung function. The study, published in Nature Genetics, was led by researchers at Karolinska Institutet in Sweden and the Institute for Molecular Medicine Finland.
Biological causes behind persistent symptoms after COVID-19 infection, known as long COVID or post-COVID, remain unclear. Common symptoms include fatigue, cognitive difficulties, and breathing problems, which can reduce quality of life.
In an international collaboration – the Long COVID Host Genetics Initiative – researchers have analysed genetic data from 6,450 long COVID patients and more than a million controls across 24 studies from 16 countries. They found a gene variant that increases the risk of long COVID by about 60 percent. The genetic association was confirmed in an independent analysis involving an additional 9,500 cases.
Impaired lung function plays a key role
The gene variant is located right next to the gene FOXP4, which is involved in lung development and lung disease.
“Our findings suggest that impaired lung function plays a key role in developing long COVID,” says Hugo Zeberg, senior lecturer at the Department of Physiology and Pharmacology, Karolinska Institutet, and one of the lead researchers of the study. “While this gene variant significantly increases risk, it’s important to recognise it as just one part of a much larger puzzle,” he continues.
“Genetic studies can provide insights into disease risk factors and are particularly powerful for diseases where the exact mechanisms remain unknown,” says Hanna Ollila, FIMM-EMBL group leader at the Institute for Molecular Medicine Finland, University of Helsinki, and researcher at the Department of Anesthesia and Center for Genomic Medicine at Massachusetts General Hospital, who co-led the study.
See the published paper for information about funding and potential conflicts of interest.
Publication: “Genome-wide association study of long COVID”, Vilma Lammi et al., Nature Genetics, online 21 May 2025, doi: 10.1038/s41588-025-02100-w.
Journal
Nature Genetics
DOI
10.1038/s41588-025-02100-w
Subject of Research
People
Article Title
Genome-wide association study of long COVID
Article Publication Date
21-May-2025
See subsequent analysis In Silico Analysis of Post-COVID-19 Condition PCC Associated SNP rs9367106 Predicts the Molecular Basis of Abnormalities in the Lungs and Brain Functions (2025)
Along with the Nature publications from the COVID-19 Host Genetics Initiative —
Mapping the human genetic architecture of COVID-19 (2021)
A first update on mapping the human genetic architecture of COVID-19 (2022)
A second update on mapping the human genetic architecture of COVID-19 (2023)
Along with the Nature publications from the COVID-19 Host Genetics Initiative —
Mapping the human genetic architecture of COVID-19 (2021)
A first update on mapping the human genetic architecture of COVID-19 (2022)
A second update on mapping the human genetic architecture of COVID-19 (2023)
wastwater
Senior Member (Voting Rights)
A genetic risk factor for the development of #LongCovid!A new study identified the first genome-wide significant association for #LongCOVID at the FOXP4 locus. 1/

wastwater
Senior Member (Voting Rights)
FOXP4 Variants Are Associated With Plateau Iris and Angle Closure Glaucoma - PMC
Angle closure glaucoma (ACG) is a common cause of adult-onset vision loss that often presents with iris abnormalities and short axial lengths. Although it is heritable, little is known about the genetic risk factors underlying this condition. We ...
Last edited:
