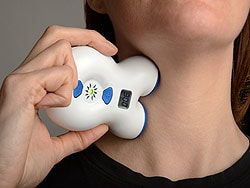
The US Food and Drug Administration (FDA) has cleared the hand-held, noninvasive vagus nerve stimulator (nVS)
gammaCore (electroCore LLC) for the treatment of migraine pain in adults, the manufacturer announced earlier today.
The new 510(k) clearance will expand the device's label from just treating episodic cluster headache pain, an indication that the
FDA approved in April 2017.
Courtesy of gammaCore
After the portable device is placed over the vagus nerve in the neck, it releases a mild electrical stimulation to the nerve's afferent fibers.
"With the FDA's decision to release gammaCore for migraine, patients now have access to an effective and safe therapy which can be self-administered to acutely treat the pain associated with migraine," Stephen D. Silberstein, MD, professor of neurology and director of the Headache Center at Thomas Jefferson University, Philadelphia, Pennsylvania, said in a news release.