V.R.T.
Senior Member (Voting Rights)
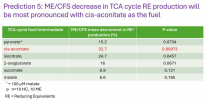
So moderate/severe disease then? That's good in a way. Less chance of having some people who have idiopathic chronic fatigue in there.I don't know about the two specific people in the images, but in the demographic data screenshot for all 13 women, for the multidimensional fatigue inventory (MFI) score it says 86.62 for cases and 32.08 for controls.
From a paper:
Interesting that the controls scored so higly for fatigue - were they sedentary controls, and did they have another disease that might cause fatigue?