Published version linked in a later post
*****
Preprint
Choroid Plexus calcification correlates with cortical microglial activation in humans: a multimodal PET, CT, MRI study
Tracy Butler, X. Hugh Wang, Gloria C. Chiang, Yi Li, Liangdong Zhou, Ke Xi, NimmiWickramasuriya, Emily Tanzi, Edward Spector, Ilker Ozsahin, Xiangling Mao, Q. RayRazlighi, Edward K. Fung, Jonathan P. Dyke, Thomas R. Maloney, Ajay Gupta, Ashish Raj, Dikoma C. Shungu, P. David Mozley, Henry Rusinek, Lidia Glodzik
Background
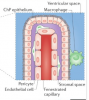
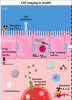
Choroid plexus (CP) within brain ventricles is well known to produce CSF. Additional important CP functions are now recognized including critical modulation of inflammation. Recent MRI studies have demonstrated CP enlargement in human diseases including Multiple Sclerosis and Alzheimers Disease, and in association with neuroinflammation measured using translocator protein (TSPO) PET. The basis of MRI—visible CP enlargement is unknown.
Purpose
Based on tissue studies demonstrating CP calcification as a common pathology associated with aging and disease, we hypothesized that previously—unmeasured calcium within CP contributes to MRI—measured CP volume, and may be more specifically associated with neuroinflammation.
Materials and Methods
We performed a retrospective analysis of PET—CT studies performed between 2013—2019 on a single scanner using the TSPO radiotracer 11C—PK11195. Subjects included controls (n=43) and patients diagnosed with several non—inflammatory neuropsychiatric conditions (n=46.) Cortical inflammation / microglial activation was quantified as nondisplaceable Binding Potential (BPnd.) CP and ventricle volume were measured using Freesurfer. CP calcium was measured semi—manually via tracing of low—dose CT acquired with PET and automatically using a new CT/MRI method. The contribution of CP calcium, CP overall volume, ventricle volume, subject age, sex and diagnosis to BPnd was assessed using linear regression.
Results
89 subjects (mean age 54+/—7 years; 52 men) were included. Fully—automated CP calcium quantification was accurate (ICC with semi—manual tracing = .98.) The significant predictors of cortical neuroinflammation were subject age (p=.002) and CP calcium volume (p=.041), but not ventricle or CP volume.
Conclusion
CP calcium volume can be accurately measured using low—dose CT acquired routinely with PET—CT. CP calcification — but not CP overall volume — was associated with cortical inflammation. Unmeasured CP calcification may be relevant to recent reports of CP enlargement in human inflammatory and other diseases. CP calcification may be a specific and relatively easily—acquired biomarker for neuroinflammation and CP pathology.
MedRxiv
*****
Preprint
Choroid Plexus calcification correlates with cortical microglial activation in humans: a multimodal PET, CT, MRI study
Tracy Butler, X. Hugh Wang, Gloria C. Chiang, Yi Li, Liangdong Zhou, Ke Xi, NimmiWickramasuriya, Emily Tanzi, Edward Spector, Ilker Ozsahin, Xiangling Mao, Q. RayRazlighi, Edward K. Fung, Jonathan P. Dyke, Thomas R. Maloney, Ajay Gupta, Ashish Raj, Dikoma C. Shungu, P. David Mozley, Henry Rusinek, Lidia Glodzik
Background
Choroid plexus (CP) within brain ventricles is well known to produce CSF. Additional important CP functions are now recognized including critical modulation of inflammation. Recent MRI studies have demonstrated CP enlargement in human diseases including Multiple Sclerosis and Alzheimers Disease, and in association with neuroinflammation measured using translocator protein (TSPO) PET. The basis of MRI—visible CP enlargement is unknown.
Purpose
Based on tissue studies demonstrating CP calcification as a common pathology associated with aging and disease, we hypothesized that previously—unmeasured calcium within CP contributes to MRI—measured CP volume, and may be more specifically associated with neuroinflammation.
Materials and Methods
We performed a retrospective analysis of PET—CT studies performed between 2013—2019 on a single scanner using the TSPO radiotracer 11C—PK11195. Subjects included controls (n=43) and patients diagnosed with several non—inflammatory neuropsychiatric conditions (n=46.) Cortical inflammation / microglial activation was quantified as nondisplaceable Binding Potential (BPnd.) CP and ventricle volume were measured using Freesurfer. CP calcium was measured semi—manually via tracing of low—dose CT acquired with PET and automatically using a new CT/MRI method. The contribution of CP calcium, CP overall volume, ventricle volume, subject age, sex and diagnosis to BPnd was assessed using linear regression.
Results
89 subjects (mean age 54+/—7 years; 52 men) were included. Fully—automated CP calcium quantification was accurate (ICC with semi—manual tracing = .98.) The significant predictors of cortical neuroinflammation were subject age (p=.002) and CP calcium volume (p=.041), but not ventricle or CP volume.
Conclusion
CP calcium volume can be accurately measured using low—dose CT acquired routinely with PET—CT. CP calcification — but not CP overall volume — was associated with cortical inflammation. Unmeasured CP calcification may be relevant to recent reports of CP enlargement in human inflammatory and other diseases. CP calcification may be a specific and relatively easily—acquired biomarker for neuroinflammation and CP pathology.
MedRxiv
Last edited by a moderator: