Ravn
Senior Member (Voting Rights)
Authors: David Fineberg, Alain Moreau, Elena K Schneider-Futschik, Christopher W. Armstrong
Abstract
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and Long COVID (LC) are increasingly recognized as debilitating postinfectious conditions that impact both individuals and society. Recent research highlights the potential of metformin, an antidiabetic agent, as a treatment for these syndromes by targeting their underlying mechanisms.
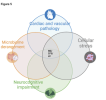
This review assesses the effectiveness of metformin in ME/CFS and LC, which involve complex dysfunctions related to cytokines, glycolysis, ATP generation, oxidative stress, gastrointestinal microbiomes, and vascular endothelial function.
Metformin, traditionally known for its antihyperglycemic properties may offer broader therapeutic benefits by influencing these pathological pathways. It works by inhibiting complexes I and IV of the electron transport chain, which reduces the strain on malfunctioning complex V and decreases the production of harmful free radicals.
Additionally, metformin’s impact on mTOR signaling could improve energy metabolism in ME/CFS and LC by downregulating an overactive but underperforming protein, thereby alleviating symptoms.
Beyond the impact on cellular metabolism, metformin has shown to have anti-inflammatory, vascular, gastrointestinal, neuroprotective and epigenetic effects.
We explore this impact of metformin and the potential role it could play to help people with ME/CFS. While metformin shows promise, it is unlikely to be a stand-alone solution. Instead, it may be part of a broader treatment strategy that includes other therapies targeting neurocognitive and autonomic impairments.
https://pubs.acs.org/doi/full/10.1021/acsptsci.5c00229
Open access
Abstract
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and Long COVID (LC) are increasingly recognized as debilitating postinfectious conditions that impact both individuals and society. Recent research highlights the potential of metformin, an antidiabetic agent, as a treatment for these syndromes by targeting their underlying mechanisms.
This review assesses the effectiveness of metformin in ME/CFS and LC, which involve complex dysfunctions related to cytokines, glycolysis, ATP generation, oxidative stress, gastrointestinal microbiomes, and vascular endothelial function.
Metformin, traditionally known for its antihyperglycemic properties may offer broader therapeutic benefits by influencing these pathological pathways. It works by inhibiting complexes I and IV of the electron transport chain, which reduces the strain on malfunctioning complex V and decreases the production of harmful free radicals.
Additionally, metformin’s impact on mTOR signaling could improve energy metabolism in ME/CFS and LC by downregulating an overactive but underperforming protein, thereby alleviating symptoms.
Beyond the impact on cellular metabolism, metformin has shown to have anti-inflammatory, vascular, gastrointestinal, neuroprotective and epigenetic effects.
We explore this impact of metformin and the potential role it could play to help people with ME/CFS. While metformin shows promise, it is unlikely to be a stand-alone solution. Instead, it may be part of a broader treatment strategy that includes other therapies targeting neurocognitive and autonomic impairments.
https://pubs.acs.org/doi/full/10.1021/acsptsci.5c00229
Open access