Now published - see post #17
Preprint
A Digital Platform with Activity Tracking for Energy Management Support in Long COVID: A Randomised Controlled Trial
Lawrence Hayes, Nilihan Sanal-Hayes, Jacqueline Mair, Antonio Dello Iacono, Joanne Ingram, Jane Ormerod, David Carless, Natlie Hilliard, Marie Mclaughlin, Rachel Meach, Nicholas Sculthorpe
Abstract
People with long COVID (LC) report worsening symptoms after activity, like post-exertional malaise (PEM) in chronic fatigue syndrome (CFS). The National Institute for Health and Care Excellence (NICE) recommends ‘energy management’ for CFS, but at the time of writing, how people with LC would respond to energy management was unknown.
In a 6-month pragmatic decentralised randomised controlled trial (RCT), we compared a just-in-time intervention to support energy management in adults with LC to standard care. Participants were randomised to receive either the ‘Pace Me’ app and a wearable activity tracker (intervention) or an app only with data entry screens (control). The intervention group received just-in-time messages on PEM management when they reached 50%, 75%, and 100% of their daily ‘activity allowance’. The primary outcome was PEM measured by the DePaul Symptom Questionnaire-Post-Exertional Malaise (DSQ-PEM).
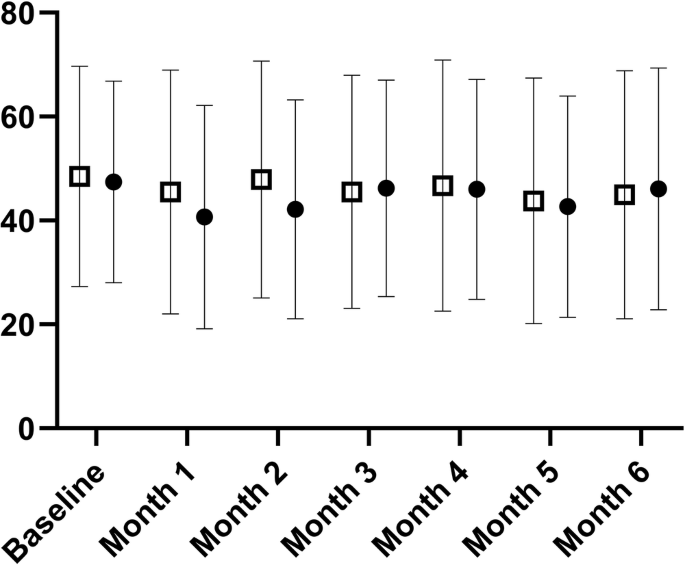
Of 368 participants assessed for eligibility, 250 participants were randomised 1:1, but 36 control and eight intervention participants were lost to follow-up. 12 control and 24 intervention participants were excluded from analysis due to missing data. 84 intervention participants and 77 control participants were analysed. There was no time by group interaction for the DSQ-PEM. The intervention group value was 48 (95% CI 44–53) pre-intervention and 46 (95% CI 41–51) post-intervention (arbitrary units). The control group value was 47 (95% CI 42–52) pre-intervention and 44 (95% CI 39–49) post-intervention (interaction effect p = 0.614, η²p = 0.002; trivial). No individual question exhibited an interaction effect (P > 0.05).
Digitally supported energy management in people with LC had no effect on PEM compared to standard care. Although the intervention had no additional effect compared to control, the substantial recovery rate in LC may have masked intervention effects. Therefore, future studies should consider this energy management framework in conditions without such recovery rates, such as CFS.
Link | PDF (Preprint: Research Square) [Open Access]
Preprint
A Digital Platform with Activity Tracking for Energy Management Support in Long COVID: A Randomised Controlled Trial
Lawrence Hayes, Nilihan Sanal-Hayes, Jacqueline Mair, Antonio Dello Iacono, Joanne Ingram, Jane Ormerod, David Carless, Natlie Hilliard, Marie Mclaughlin, Rachel Meach, Nicholas Sculthorpe
Abstract
People with long COVID (LC) report worsening symptoms after activity, like post-exertional malaise (PEM) in chronic fatigue syndrome (CFS). The National Institute for Health and Care Excellence (NICE) recommends ‘energy management’ for CFS, but at the time of writing, how people with LC would respond to energy management was unknown.
In a 6-month pragmatic decentralised randomised controlled trial (RCT), we compared a just-in-time intervention to support energy management in adults with LC to standard care. Participants were randomised to receive either the ‘Pace Me’ app and a wearable activity tracker (intervention) or an app only with data entry screens (control). The intervention group received just-in-time messages on PEM management when they reached 50%, 75%, and 100% of their daily ‘activity allowance’. The primary outcome was PEM measured by the DePaul Symptom Questionnaire-Post-Exertional Malaise (DSQ-PEM).
Of 368 participants assessed for eligibility, 250 participants were randomised 1:1, but 36 control and eight intervention participants were lost to follow-up. 12 control and 24 intervention participants were excluded from analysis due to missing data. 84 intervention participants and 77 control participants were analysed. There was no time by group interaction for the DSQ-PEM. The intervention group value was 48 (95% CI 44–53) pre-intervention and 46 (95% CI 41–51) post-intervention (arbitrary units). The control group value was 47 (95% CI 42–52) pre-intervention and 44 (95% CI 39–49) post-intervention (interaction effect p = 0.614, η²p = 0.002; trivial). No individual question exhibited an interaction effect (P > 0.05).
Digitally supported energy management in people with LC had no effect on PEM compared to standard care. Although the intervention had no additional effect compared to control, the substantial recovery rate in LC may have masked intervention effects. Therefore, future studies should consider this energy management framework in conditions without such recovery rates, such as CFS.
Link | PDF (Preprint: Research Square) [Open Access]
Last edited by a moderator: