Abstract
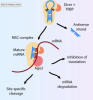
Neuropathic pain is a debilitating condition affecting around 8% of the adult population in the UK. The pathophysiology is complex and involves a wide range of processes, including alteration of neuronal excitability and synaptic transmission, dysregulated intracellular signalling and activation of pro-inflammatory immune and glial cells. In the past 15 years, multiple miRNAs–small non-coding RNA–have emerged as regulators of neuropathic pain development. They act by binding to target mRNAs and preventing the translation into proteins. Due to their short sequence (around 22 nucleotides in length), they can have hundreds of targets and regulate several pathways.
Several studies on animal models have highlighted numerous miRNAs that play a role in neuropathic pain development at various stages of the nociceptive pathways, including neuronal excitability, synaptic transmission, intracellular signalling and communication with non-neuronal cells. Studies on animal models do not always translate in the clinic; fewer studies on miRNAs have been performed involving human subjects with neuropathic pain, with differing results depending on the specific aetiology underlying neuropathic pain. Further studies using human tissue and liquid samples (serum, plasma, saliva) will help highlight miRNAs that are relevant to neuropathic pain diagnosis or treatment, as biomarkers or potential drug targets.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10045079/
Neuropathic pain is a debilitating condition affecting around 8% of the adult population in the UK. The pathophysiology is complex and involves a wide range of processes, including alteration of neuronal excitability and synaptic transmission, dysregulated intracellular signalling and activation of pro-inflammatory immune and glial cells. In the past 15 years, multiple miRNAs–small non-coding RNA–have emerged as regulators of neuropathic pain development. They act by binding to target mRNAs and preventing the translation into proteins. Due to their short sequence (around 22 nucleotides in length), they can have hundreds of targets and regulate several pathways.
Several studies on animal models have highlighted numerous miRNAs that play a role in neuropathic pain development at various stages of the nociceptive pathways, including neuronal excitability, synaptic transmission, intracellular signalling and communication with non-neuronal cells. Studies on animal models do not always translate in the clinic; fewer studies on miRNAs have been performed involving human subjects with neuropathic pain, with differing results depending on the specific aetiology underlying neuropathic pain. Further studies using human tissue and liquid samples (serum, plasma, saliva) will help highlight miRNAs that are relevant to neuropathic pain diagnosis or treatment, as biomarkers or potential drug targets.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10045079/