Cybergreen91
Established Member
YouTube Explanation:
Link to Figures:
https://docs.google.com/document/d/1Dwanor7_78l0Wej1DgYcCy0aScdKruimfGi4IObEmSo/edit?usp=sharing
Environmental science is an area of critical importance that is frequently overlooked in medical research, despite the environment’s disproportionately large influence on our health. There has been a significant increase in the reported incidence of chronic illness (from 7.5% in 1930[1] to 60% in 2020[2]). An elevation in environmental toxins are likely responsible for a substantial portion of that increase. There is also a need for a deeper understanding of causation, with pharmaceuticals proving to be ineffective for diseases such as ME/CFS, and carrying high side-effect profiles. In this video, I’ve shared my personal journey of falling ill due to exposure to hazardous chemicals. My personal experience has led me to become frustrated with the lack of research on many of these severe illnesses impacting millions. I had ME/CFS for 4 years before realizing that I had environmental sensitivities driving my illness. After moving to a new apartment in 2021, I experienced health issues related to wood varnish, mold exposure, and paint despite having never previously noticed. It became overwhelmingly obvious that this “Chronic Fatigue Syndrome” was really driven by environmental causes. I believe my experiences are applicable to the millions of people who have developed long COVID and ME/CFS from a viral event. This is because even if environmental triggers did not initiate their illness, there may be environmental components perpetuating their symptoms.
Through my efforts in this video, I aim to use my own experiences and research to challenge the traditional medical view that often treats patients as if they exist in a vacuum. Instead, I seek to emphasize the constant influence of the environment on human health. I reject the term "dysfunction," asserting that chronic illnesses are a persisting state of damage rather than the result of a pre-existing physiological dysfunction. I believe that the combination of the type of exposure and an individual’s biology/genetics evoke a particular response to that damage, which ultimately determines what disease is developed. Specifically, I make the case for ME/CFS and MCS being a “hypoproliferative” state (i.e. a state where normative function has been lost (Fig. 1). I argue that the lack of a cure, despite numerous available medications prove the absence of an underlying physiological dysfunction, because in this scenario the body is operating and compensating to the best of its ability. Consider the following analogy about farming: if you're a farmer and you're growing some plants in a field and one day you see all of your plants are sick and dying, do you conclude that these plants have some “genetic, mitochondrial, psychosomatic disorder”? Or are you just a bad farmer, and not giving them enough food, water, sunlight and protecting them from disease and pests? Why is it that when it comes to plants, we accept that everything is environmental, but when it comes to people it never is?
I’d like to propose a mechanism by which factors in our environments can either initiate or perpetuate symptoms in chronic illnesses, such as Chronic Fatigue Syndrome. The first phase of the disease induction is about epithelial barrier damage. The body has many protective barriers to prevent toxins and contaminants from entering, not just the more commonly known blood-brain barrier. The role of environmental toxins contributing to damage of these barriers is not a new concept, and has been previously associated with conditions like allergies and asthma[3]. However, it would be new to connect it to multiple chemical sensitivity (MCS) and chronic fatigue syndrome.
The epithelial barrier could theoretically be damaged by many factors, but three main ones to focus on are: chemical exposures (pesticides, paint, etc.), viral infections (such as SARS-CoV-2 affecting lung epithelial cells)[4], and connective tissue disorders disrupting cell orientation. Environmental sensitivities are not necessarily triggered by acute exposures but can result from chronic exposure to various household/environmental contaminants, leading to symptoms without the person being consciously aware of them.
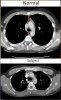
I have proposed a model to explain how someone can develop multiple chemical sensitivity (MCS) or experience worsening symptoms over time (Fig. 2). In the model, a barrier damaging event will reduce the number of healthy epithelial cells. As exposure levels remain the same, the number of buffering barrier cells decrease, leading to increased toxicity and damage, creating a cycle of worsening symptoms. There might be evidence of this damage seen in chest CT images, where I’ve highlighted the compression in the area where the thymus is located (Fig 3.). Damage from VOCs could be eliminating these barrier cells act as a buffer. There may even be a direct impact of this, as compression in the chest could lead to thoracic outlet syndrome and affect vagus nerve function. Adrenergic autoantibodies found in ME/CFS and POTS patients have been a large area of focus the past several years. A prior study[5] proposed a link between barrier permeability in the gut and the production of these autoantibodies, suggesting that microbial molecules translocated from the GI tract may trigger autoimmunity. This would imply that these adrenergic autoantibodies are actually a marker of intestinal permeability.
From experience, I can say there are three main types of exposures that must be avoided: VOCs (which can cause a stimulated feeling and reduced ability to rest/relax), mold (which can cause inflammation and flu-like symptoms) and insoluble nanoparticles (which can lead to chronic fatigue and pain). The condition is a state of damage, not an allergic reaction or autoimmune disease, although immunological abnormalities may occur as a result of exposure. It’s important to acknowledge that barrier permeability precedes mast cell activation, which challenges the common belief that mass cell activation is a prerequisite for chemical sensitivity, chronic fatigue, or fibromyalgia. To emphasize the physiological nature of the condition, I shared a photo of skin damage caused by adhesive from a standard Band-Aid (Fig. 4). This is a real physiological toxicity, not a problem with neurosensitization, highlighting the importance of recognizing physical signs of intolerance.
In the second phase of this mechanism, I’d like to introduce insoluble nanoparticles as a novel category of exposure contributing to chronic fatigue syndrome, POTS, and fibromyalgia. I will be referencing titanium dioxide specifically; however, the category covers other inert particles like silica, calcium carbonate, and aluminum oxide. Theseparticles are minuscule, much smaller than a grain of sand, with the primary source of inhalation exposure coming from matte white paint used in buildings and homes. I believe these nanoparticles can clog, tear, and impede blood flow, leading to pain and fatigue. When patients describe the feeling of “having cement in their blood”, they might not actually be far off. These particles, while not inherently toxic[6], can be abrasive and lead to damage of connective tissue and nerves. There is widespread use of titanium dioxide in various products such as paint, toothpaste, tattoos, sunscreen, food items, paper, and pills. Although it serves as a whitening agent, it’s also an extremely hard abrasive in toothpaste. This scrubbing mechanism, in my view, adds to the narrative that there is physical damage to connective tissue and nerves.
To highlight the reasons for focusing on titanium dioxide, I’d like to note that it’s very commonly used and has a relatively long half-life[7]. The half-life also depends on exposure levels, with larger exposure events leading to extended half-lives[8]. This means the substance potentially stays in the body for years, or possibly even at relevant levels over a lifetime. Even if these nanoparticles are slowly removed from the body, exposure is so common that they are still likely to accumulate. Additionally, even if the absolute number of nanoparticles drop, damage may continue to accumulate. Although the gastrointestinal absorption of these particles is low[9], the extremely fast rate of respiratory absorption remains a concern[10]. The airborne nanoparticles from matte white paint are too light to ever settle on the ground, which makes areas of contamination very long lasting.
Nanoparticles have been shown to induce a “chronic fatigue syndrome-like illness” in the literature, which reports of passive behavior, loss of appetite, tremor, and lethargy, in the absence of major structural or biomarker changes at lower doses[11]. They appear to exert a number of multi-system effects from the literature, including damage to the epithelial barrier[12], impairing lymphatics[13], increasing oxidative stress[14], and affecting liver function[15], all of which may contribute to further susceptibilities to environmental
exposures. Drawing on personal experiences, damage appears to be indiscriminate, affecting connective tissue, affecting skin, nerves, muscles, and blood vessels. Mechanical damage seems to be the most logical explanation for how so many different types of tissue are affected.
Connecting what is seen in the literature to my own clinical manifestations, titanium dioxide exposure has been known to lead to a separation of muscle fibers[16], due to disruption of junction proteins, which likely correlate with striations seen in skin shortly after the exposure event (Fig. 5). You can also note blood vessel leakage, visible as red dots on the arms, linking it to titanium dioxide's tendency to cause endothelial cell leakiness[17] (Fig. 6). We can also draw inferences on exposures in the white matter hyperintensities seen in MRI, attributing them to mechanical stretching[18]. Additionally, the impaired peripheral oxygen extraction in CFS[19] could be explained by nanoparticle blockage of interstitial spaces.
Lymphatic dysfunction is a big part of CFS, and I can refer to Dr. Raymond Perrin's studies on engorged lymphatics in CFS patients[20] (Fig. 7). Additionally, there is a study involving rats exposed to titanium dioxide, showing its accumulation in lymph nodes[21], which would potentially disrupt lymphatic flow. There is also literature evidence of metabolic disturbances from titanium dioxide exposure, with one such study showing low metabolic biomarkers across the board in exposed rats[22] (Fig. 8).
A major aspect of focus in Long COVID and ME/CFS research lately has been around amyloid-fibrin microclots. In my nail-fold capillaroscopy analyses (Fig. 9), I very frequently observed lots of “black dots” wherever the microhemorrhages were, which may actually be microclots themselves. I think that there is a possibility that these microclots could be linked to titanium dioxide exposure. What if these nanoparticles were nucleating these microclots? I found one study showing that titanium dioxide permanently binds to fibrinogen[23]. If titanium dioxide particles are binding multiple fibrin/fibrinogen molecules, it would explain the formation of nanoparticles in the absence of other clotting factors.
Furthermore, I conducted novel research into the mechanisms of ME/CFS using transmission electron microscopy (TEM) imaging of a punch biopsy, aiming to explore subcellular details related to titanium dioxide exposure (Fig. 10). Seen were abnormalities in Schwann cells and keratinocytes (responsible for forming the epithelial barrier). In the Schwann cells, I observed giant masses of black dots (likely glycogen deposits), and white clumps (potentially indicating endoplasmic reticulum dysfunction). I consulted with about a dozen top nerve morphologists who specialize in this type of imaging and confirmed the abnormality. Of greatest concern, were pockets formed in the myelin. The pockets were unique, and unrelated to other myelinated conditions as seen in MS or dense degeneration from aging, because the pockets went through multiple layers of myelin. Thinking conceptually, the most logical cause of this type of damage seems to be something mechanical, either a puncture or tear. With the culmination of these neurological abnormalities in the absence of a reduced nerve count, we can conclude that there is a great need for a new approach to neuropathy testing, with TEM being a valuable tool for assessing damage. Simple nerve density counts from punch biopsies are not sufficient or capable of measuring these types of damage. There is also a possibility of direct evidence of nanoparticles seen in the sample, though further testing is required (Fig. 11).
Instead of spending countless dollars and years outlining every biomarker change, we need to seek immediate benefits in quality of life for those suffering from chronic illnesses. I can share my insights from a survey I conducted on beneficial treatments (Fig. 12), which showed some options for potentially beneficial therapies such as Larazotide acetate, fecal transplants, BPC 157, IV saline, oxymatrine, HBOT, and IVIG. We also need to avoid prescribing commonly issued medications that patients report as harmful. Conducting surveys like this one can quickly identify potential treatment options that lead to a benefit in quality of life for these patients. From another survey, I saw an approximately 80% overlap between self-identified CFS and MCS, which may actually be lower than the actual number due to inability to identify complex environmental triggers. However, knowing the mechanism in which patients are sick doesn’t mean I have a cure. However, hypothetically I think that a treatment regimen would likely constitute repairing the epithelial barrier by avoiding VOC exposure and/or nebulization of placental stem cells, removing titanium dioxide nanoparticles by increasing bioavailability by lymphatic massage and removing them via blood draws, and generally improving regenerative capacity.
In conclusion, I urge other researchers to consider the mechanisms that I outlined around barrier damage and mechanical damage/clogging from insoluble nanoparticles. I’d also like to emphasize the importance of avoiding harmful exposures and minimizing exertion for general health. There is a need for environmental science to play a crucial role in medicine. There is very little awareness in the research community regarding environmental role in chronic illness and epithelial barrier damage, and no one conducting any research on titanium dioxide exposure relating to it. I hope that the evidence I have provided and testimony I have given is enough to spur researchers to investigate this area, because no matter how hard we work, we will never find a cure if we aren’t investigating the right areas.
Link to Figures:
https://docs.google.com/document/d/1Dwanor7_78l0Wej1DgYcCy0aScdKruimfGi4IObEmSo/edit?usp=sharing
Environmental science is an area of critical importance that is frequently overlooked in medical research, despite the environment’s disproportionately large influence on our health. There has been a significant increase in the reported incidence of chronic illness (from 7.5% in 1930[1] to 60% in 2020[2]). An elevation in environmental toxins are likely responsible for a substantial portion of that increase. There is also a need for a deeper understanding of causation, with pharmaceuticals proving to be ineffective for diseases such as ME/CFS, and carrying high side-effect profiles. In this video, I’ve shared my personal journey of falling ill due to exposure to hazardous chemicals. My personal experience has led me to become frustrated with the lack of research on many of these severe illnesses impacting millions. I had ME/CFS for 4 years before realizing that I had environmental sensitivities driving my illness. After moving to a new apartment in 2021, I experienced health issues related to wood varnish, mold exposure, and paint despite having never previously noticed. It became overwhelmingly obvious that this “Chronic Fatigue Syndrome” was really driven by environmental causes. I believe my experiences are applicable to the millions of people who have developed long COVID and ME/CFS from a viral event. This is because even if environmental triggers did not initiate their illness, there may be environmental components perpetuating their symptoms.
Through my efforts in this video, I aim to use my own experiences and research to challenge the traditional medical view that often treats patients as if they exist in a vacuum. Instead, I seek to emphasize the constant influence of the environment on human health. I reject the term "dysfunction," asserting that chronic illnesses are a persisting state of damage rather than the result of a pre-existing physiological dysfunction. I believe that the combination of the type of exposure and an individual’s biology/genetics evoke a particular response to that damage, which ultimately determines what disease is developed. Specifically, I make the case for ME/CFS and MCS being a “hypoproliferative” state (i.e. a state where normative function has been lost (Fig. 1). I argue that the lack of a cure, despite numerous available medications prove the absence of an underlying physiological dysfunction, because in this scenario the body is operating and compensating to the best of its ability. Consider the following analogy about farming: if you're a farmer and you're growing some plants in a field and one day you see all of your plants are sick and dying, do you conclude that these plants have some “genetic, mitochondrial, psychosomatic disorder”? Or are you just a bad farmer, and not giving them enough food, water, sunlight and protecting them from disease and pests? Why is it that when it comes to plants, we accept that everything is environmental, but when it comes to people it never is?
I’d like to propose a mechanism by which factors in our environments can either initiate or perpetuate symptoms in chronic illnesses, such as Chronic Fatigue Syndrome. The first phase of the disease induction is about epithelial barrier damage. The body has many protective barriers to prevent toxins and contaminants from entering, not just the more commonly known blood-brain barrier. The role of environmental toxins contributing to damage of these barriers is not a new concept, and has been previously associated with conditions like allergies and asthma[3]. However, it would be new to connect it to multiple chemical sensitivity (MCS) and chronic fatigue syndrome.
The epithelial barrier could theoretically be damaged by many factors, but three main ones to focus on are: chemical exposures (pesticides, paint, etc.), viral infections (such as SARS-CoV-2 affecting lung epithelial cells)[4], and connective tissue disorders disrupting cell orientation. Environmental sensitivities are not necessarily triggered by acute exposures but can result from chronic exposure to various household/environmental contaminants, leading to symptoms without the person being consciously aware of them.
I have proposed a model to explain how someone can develop multiple chemical sensitivity (MCS) or experience worsening symptoms over time (Fig. 2). In the model, a barrier damaging event will reduce the number of healthy epithelial cells. As exposure levels remain the same, the number of buffering barrier cells decrease, leading to increased toxicity and damage, creating a cycle of worsening symptoms. There might be evidence of this damage seen in chest CT images, where I’ve highlighted the compression in the area where the thymus is located (Fig 3.). Damage from VOCs could be eliminating these barrier cells act as a buffer. There may even be a direct impact of this, as compression in the chest could lead to thoracic outlet syndrome and affect vagus nerve function. Adrenergic autoantibodies found in ME/CFS and POTS patients have been a large area of focus the past several years. A prior study[5] proposed a link between barrier permeability in the gut and the production of these autoantibodies, suggesting that microbial molecules translocated from the GI tract may trigger autoimmunity. This would imply that these adrenergic autoantibodies are actually a marker of intestinal permeability.
From experience, I can say there are three main types of exposures that must be avoided: VOCs (which can cause a stimulated feeling and reduced ability to rest/relax), mold (which can cause inflammation and flu-like symptoms) and insoluble nanoparticles (which can lead to chronic fatigue and pain). The condition is a state of damage, not an allergic reaction or autoimmune disease, although immunological abnormalities may occur as a result of exposure. It’s important to acknowledge that barrier permeability precedes mast cell activation, which challenges the common belief that mass cell activation is a prerequisite for chemical sensitivity, chronic fatigue, or fibromyalgia. To emphasize the physiological nature of the condition, I shared a photo of skin damage caused by adhesive from a standard Band-Aid (Fig. 4). This is a real physiological toxicity, not a problem with neurosensitization, highlighting the importance of recognizing physical signs of intolerance.
In the second phase of this mechanism, I’d like to introduce insoluble nanoparticles as a novel category of exposure contributing to chronic fatigue syndrome, POTS, and fibromyalgia. I will be referencing titanium dioxide specifically; however, the category covers other inert particles like silica, calcium carbonate, and aluminum oxide. Theseparticles are minuscule, much smaller than a grain of sand, with the primary source of inhalation exposure coming from matte white paint used in buildings and homes. I believe these nanoparticles can clog, tear, and impede blood flow, leading to pain and fatigue. When patients describe the feeling of “having cement in their blood”, they might not actually be far off. These particles, while not inherently toxic[6], can be abrasive and lead to damage of connective tissue and nerves. There is widespread use of titanium dioxide in various products such as paint, toothpaste, tattoos, sunscreen, food items, paper, and pills. Although it serves as a whitening agent, it’s also an extremely hard abrasive in toothpaste. This scrubbing mechanism, in my view, adds to the narrative that there is physical damage to connective tissue and nerves.
To highlight the reasons for focusing on titanium dioxide, I’d like to note that it’s very commonly used and has a relatively long half-life[7]. The half-life also depends on exposure levels, with larger exposure events leading to extended half-lives[8]. This means the substance potentially stays in the body for years, or possibly even at relevant levels over a lifetime. Even if these nanoparticles are slowly removed from the body, exposure is so common that they are still likely to accumulate. Additionally, even if the absolute number of nanoparticles drop, damage may continue to accumulate. Although the gastrointestinal absorption of these particles is low[9], the extremely fast rate of respiratory absorption remains a concern[10]. The airborne nanoparticles from matte white paint are too light to ever settle on the ground, which makes areas of contamination very long lasting.
Nanoparticles have been shown to induce a “chronic fatigue syndrome-like illness” in the literature, which reports of passive behavior, loss of appetite, tremor, and lethargy, in the absence of major structural or biomarker changes at lower doses[11]. They appear to exert a number of multi-system effects from the literature, including damage to the epithelial barrier[12], impairing lymphatics[13], increasing oxidative stress[14], and affecting liver function[15], all of which may contribute to further susceptibilities to environmental
exposures. Drawing on personal experiences, damage appears to be indiscriminate, affecting connective tissue, affecting skin, nerves, muscles, and blood vessels. Mechanical damage seems to be the most logical explanation for how so many different types of tissue are affected.
Connecting what is seen in the literature to my own clinical manifestations, titanium dioxide exposure has been known to lead to a separation of muscle fibers[16], due to disruption of junction proteins, which likely correlate with striations seen in skin shortly after the exposure event (Fig. 5). You can also note blood vessel leakage, visible as red dots on the arms, linking it to titanium dioxide's tendency to cause endothelial cell leakiness[17] (Fig. 6). We can also draw inferences on exposures in the white matter hyperintensities seen in MRI, attributing them to mechanical stretching[18]. Additionally, the impaired peripheral oxygen extraction in CFS[19] could be explained by nanoparticle blockage of interstitial spaces.
Lymphatic dysfunction is a big part of CFS, and I can refer to Dr. Raymond Perrin's studies on engorged lymphatics in CFS patients[20] (Fig. 7). Additionally, there is a study involving rats exposed to titanium dioxide, showing its accumulation in lymph nodes[21], which would potentially disrupt lymphatic flow. There is also literature evidence of metabolic disturbances from titanium dioxide exposure, with one such study showing low metabolic biomarkers across the board in exposed rats[22] (Fig. 8).
A major aspect of focus in Long COVID and ME/CFS research lately has been around amyloid-fibrin microclots. In my nail-fold capillaroscopy analyses (Fig. 9), I very frequently observed lots of “black dots” wherever the microhemorrhages were, which may actually be microclots themselves. I think that there is a possibility that these microclots could be linked to titanium dioxide exposure. What if these nanoparticles were nucleating these microclots? I found one study showing that titanium dioxide permanently binds to fibrinogen[23]. If titanium dioxide particles are binding multiple fibrin/fibrinogen molecules, it would explain the formation of nanoparticles in the absence of other clotting factors.
Furthermore, I conducted novel research into the mechanisms of ME/CFS using transmission electron microscopy (TEM) imaging of a punch biopsy, aiming to explore subcellular details related to titanium dioxide exposure (Fig. 10). Seen were abnormalities in Schwann cells and keratinocytes (responsible for forming the epithelial barrier). In the Schwann cells, I observed giant masses of black dots (likely glycogen deposits), and white clumps (potentially indicating endoplasmic reticulum dysfunction). I consulted with about a dozen top nerve morphologists who specialize in this type of imaging and confirmed the abnormality. Of greatest concern, were pockets formed in the myelin. The pockets were unique, and unrelated to other myelinated conditions as seen in MS or dense degeneration from aging, because the pockets went through multiple layers of myelin. Thinking conceptually, the most logical cause of this type of damage seems to be something mechanical, either a puncture or tear. With the culmination of these neurological abnormalities in the absence of a reduced nerve count, we can conclude that there is a great need for a new approach to neuropathy testing, with TEM being a valuable tool for assessing damage. Simple nerve density counts from punch biopsies are not sufficient or capable of measuring these types of damage. There is also a possibility of direct evidence of nanoparticles seen in the sample, though further testing is required (Fig. 11).
Instead of spending countless dollars and years outlining every biomarker change, we need to seek immediate benefits in quality of life for those suffering from chronic illnesses. I can share my insights from a survey I conducted on beneficial treatments (Fig. 12), which showed some options for potentially beneficial therapies such as Larazotide acetate, fecal transplants, BPC 157, IV saline, oxymatrine, HBOT, and IVIG. We also need to avoid prescribing commonly issued medications that patients report as harmful. Conducting surveys like this one can quickly identify potential treatment options that lead to a benefit in quality of life for these patients. From another survey, I saw an approximately 80% overlap between self-identified CFS and MCS, which may actually be lower than the actual number due to inability to identify complex environmental triggers. However, knowing the mechanism in which patients are sick doesn’t mean I have a cure. However, hypothetically I think that a treatment regimen would likely constitute repairing the epithelial barrier by avoiding VOC exposure and/or nebulization of placental stem cells, removing titanium dioxide nanoparticles by increasing bioavailability by lymphatic massage and removing them via blood draws, and generally improving regenerative capacity.
In conclusion, I urge other researchers to consider the mechanisms that I outlined around barrier damage and mechanical damage/clogging from insoluble nanoparticles. I’d also like to emphasize the importance of avoiding harmful exposures and minimizing exertion for general health. There is a need for environmental science to play a crucial role in medicine. There is very little awareness in the research community regarding environmental role in chronic illness and epithelial barrier damage, and no one conducting any research on titanium dioxide exposure relating to it. I hope that the evidence I have provided and testimony I have given is enough to spur researchers to investigate this area, because no matter how hard we work, we will never find a cure if we aren’t investigating the right areas.