John Mac
Senior Member (Voting Rights)
Abstract
Chronic fatigue syndrome (CFS) patients often suffer from severe muscle pain and an inability to exercise due to muscle fatigue.
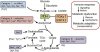
It has previously been shown that CFS skeletal muscle cells have lower levels of ATP and have AMP-activated protein kinase dysfunction.
This study outlines experiments looking at the utilisation of different substrates by skeletal muscle cells from CFS patients (n = 9) and healthy controls (n = 11) using extracellular flux analysis.
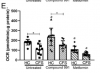
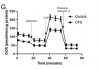
Results show that CFS skeletal muscle cells are unable to utilise glucose to the same extent as healthy control cells.
CFS skeletal muscle cells were shown to oxidise galactose and fatty acids normally, indicating that the bioenergetic dysfunction lies upstream of the TCA cycle.
The dysfunction in glucose oxidation is similar to what has previously been shown in blood cells from CFS patients.
The consistency of cellular bioenergetic dysfunction in different cell types supports the hypothesis that CFS is a systemic disease.
The retention of bioenergetic defects in cultured cells indicates that there is a genetic or epigenetic component to the disease.
This is the first study to use cells derived from skeletal muscle biopsies in CFS patients and healthy controls to look at cellular bioenergetic function in whole cells.
https://www.nature.com/articles/s41598-020-75406-w