Dolphin
Senior Member (Voting Rights)
Free fulltext:
Research Article
,
Mari Gamme Sollie
,
Harald Andre Torp
&
Dag Gundersen Storla
Received 24 Feb 2025, Accepted 19 Sep 2025, Published online: 01 Oct 2025
ABSTRACT
KEYWORDS:
Research Article
Specialised care for severely affected ME/CFS patients
Ola Didrik Saugstad,
Mari Gamme Sollie
,
Harald Andre Torp
&
Dag Gundersen Storla
Received 24 Feb 2025, Accepted 19 Sep 2025, Published online: 01 Oct 2025
ABSTRACT
Introduction
A specialised care unit for severely and very severely ill ME/CFS patients opened in 2021. The results from the first 3 years are reported.Methods
People with ME/CFS who were diagnosed according to the Canadian Consensus Criteria, who are aged 18 or above with severe or very severe ME/CFS according to the UK NICE guidelines, are eligible to stay at Røysumtunet. The study design is a retrospective review of medical records.Results
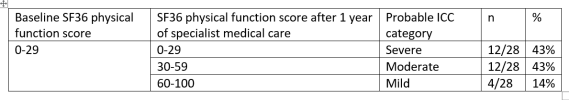
Between June 2021 and June 2024, 24 ME/CFS patients, 20 women and 4 men with a confirmed diagnosis of ME, were admitted to the unit for stays of at least 3 months. Seventeen were very severely affected and 7 were severely affected. Ages ranged from 18 to 68 years, with mean (SD) 37.5 (12.8) years. Seven patients showed significant improvement (p < 0.01), and five others showed some improvement. In total 50% improved (p < 0.01). Patients who improved were borderline significantly younger than those who did not, with a mean age of 30.3 (SD 12.6) years compared to 39.8 (SD 11.8) years (p = 0.06). The mean duration of disease was 2.3 (1.3) years for those who improved versus 6.7 (3.9) years for those who did not improve (p < 0.05).Conclusion
This is the first report of a specialised care unit for the most severely ill ME/CFS patients. Fifty per cent of patients showed significant or partial improvement. The mechanisms behind these improvements are discussed but require further exploration in future studies.KEYWORDS: