Mij
Senior Member (Voting Rights)
Authors:
Fabricia L. Fontes-Dantas et al
Highlights
Cognitive dysfunction is often reported in patients with post-COVID, but its underlying mechanisms are not completely understood. Evidence suggests that SARS-CoV-2 Spike protein or its fragments are released from the cells during infection, reaching different tissues, including the CNS, irrespective of the presence of the viral RNA.
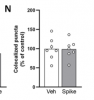
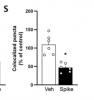
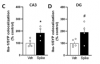
Here, we demonstrate that brain infusion of Spike protein in mice has a late impact on cognitive function, recapitulating post-COVID syndrome. We also show that neuroinflammation and hippocampal microgliosis mediates Spike-induced memory dysfunction via complement-dependent engulfment of synapses. Genetic or pharmacological blockage of TLR4 signaling protects animals against synapse elimination and memory dysfunction induced by Spike brain infusion.
Accordingly, in a cohort of 86 patients recovered from mild COVID-19, the genotype GG TLR4 -2604G>A (rs10759931) is associated with poor cognitive outcome.
These results identify TLR4 as a key target to investigate the long-term cognitive dysfunction after COVID infection both in humans and rodents.
https://www.cell.com/cell-reports/fulltext/S2211-1247(23)00200-0
Fabricia L. Fontes-Dantas et al
Highlights
- Spike protein infusion into mouse brain induces late cognitive dysfunction
- Spike protein induces late hippocampal microgliosis and synapse loss
- Blockage of TLR4 renders mice resistant to Spike-induced cognitive dysfunction
- TLR4-2604G>A GG genotype was related to poor cognitive outcomes in COVID-19 patients
Cognitive dysfunction is often reported in patients with post-COVID, but its underlying mechanisms are not completely understood. Evidence suggests that SARS-CoV-2 Spike protein or its fragments are released from the cells during infection, reaching different tissues, including the CNS, irrespective of the presence of the viral RNA.
Here, we demonstrate that brain infusion of Spike protein in mice has a late impact on cognitive function, recapitulating post-COVID syndrome. We also show that neuroinflammation and hippocampal microgliosis mediates Spike-induced memory dysfunction via complement-dependent engulfment of synapses. Genetic or pharmacological blockage of TLR4 signaling protects animals against synapse elimination and memory dysfunction induced by Spike brain infusion.
Accordingly, in a cohort of 86 patients recovered from mild COVID-19, the genotype GG TLR4 -2604G>A (rs10759931) is associated with poor cognitive outcome.
These results identify TLR4 as a key target to investigate the long-term cognitive dysfunction after COVID infection both in humans and rodents.
https://www.cell.com/cell-reports/fulltext/S2211-1247(23)00200-0