1:30:09 Michela Locci–Lymph Node Immune Responses in Long COVID
Main takeaways: This group has found differences in germinal center B cells (GC is involved in selecting and proliferating the best B cells) in long COVID. As opposed to controls, where proportion of B cells specific to SARS-CoV-2 in the germinal centers decreased over time after infection, in long COVID they increased, but still reached much lower levels. Numbers of total and SARS-CoV-2 specific B cells in the germinal centers were lower in LC. There were also fewer SARS-CoV-2 specific antibodies in the body, and lowered ability to neutralize the virus with antibodies. In the transcriptome of the B cells, they found some differences between groups, including differences related to interferon.
----------------------------------------------------------------------------------------
Locci's lab is interested in germinal centers, which are structures in lymphoid organs, such as lymph nodes and the spleen, which are important in B cell development. After an infection, the B cells most effective at binding to an antigen such as SARS-CoV-2 are selected here to be used in the immune response. The highest quality B cells can then differentiate into plasma cells, which can release antibodies to fight an infection. Germinal center B cells can also differentiate into memory B cells, which stick around after the infection to detect if it comes back, and if it does, they differentiate into plasma cells to mount a fast response. (Going by how I understood what she said and a quick skim of Wikipedia, might not be 100% accurate.)
Prior studies have suggested differences in B cell responses in long COVID.

This group hypothesizes that there is altered germinal center activity in LC, possibly releasing B cells which don't work as well, for example by not binding as well to the virus.
They have previously studied germinal center responses to vaccination by giving a vaccine in the arm, then using a needle (using process called fine needle aspiration, or FNA) to extract some cells from the nearby lymph node under the arm.
The current study is instead looking at the lymph nodes in the neck, because these are more involved in infections in the throat, where SARS-CoV-2 is often at high levels.
The study is testing 16 convalescent (COVID recovered) and 23 with long COVID, including many with fatigue and cognitive dysfunction. They are mainly looking at two time points: T1 is anywhere from 3 weeks to 6 months post-infection, and T2 is anywhere from 6 months to around a year post-infection.
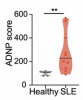
They found that in convalescent participants a high proportion of germinal center B cells were specific to SARS-CoV-2 at T1, and these are nearly gone at T2. In contrast, in those with long COVID, the proportion of such B cells was much lower at T1 compared to the healthy group, and instead of decreasing at T2, the proportion of these cells increased. Even after increasing, the level is still much lower than the peak in controls.

They also looked at the levels of SARS-CoV-2 antibodies over time. They found that these antibody levels were slightly lower soon after infection in LC, and the levels decreased faster over time than in recovered participants.

They looked at the ability of antibodies to neutralize (neutralizing antibodies attach to the virus to interfere with its function) SARS-CoV-2 in vitro (outside the body), and found a reduction of these types of antibodies at both time points compared to controls.

And they found a correlation in controls, where the larger the germinal center response (proportion of B cells specific to SARS-CoV-2 in GC), the more neutralizing antibodies were detected. But no correlation between these two metrics was found in LC.

Also looked at clonal size of total germinal center B cells and GC B cells specific to the virus (someone correct me if I'm wrong, but I think this basically means numbers of these cells in the germinal center). Found that for both measurements, and at both time points, the clonal size for LC was always lower. They think this is related to the mechanism of B cell selection, so they are digging more into this currently.

Mentioned that transcriptomics of the memory B cells (looking at RNA in the cell; highlights which proteins are being made) showed some differences, including related to interferon.
They plan to continue to test whether long COVID B cells have less ability to bind to the virus, and whether altered B cell responses are associated with viral persistence.