Perivascular spaces and their role in neuroinflammation
Benjamin V. Ineichen; Serhat V. Okar; Steven T. Proulx; Britta Engelhardt; Hans Lassmann; Daniel S. Reich
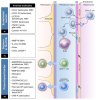
It is uncontested that perivascular spaces play critical roles in maintaining homeostasis and priming neuroinflammation. However, despite more than a century of intense research on perivascular spaces, many open questions remain about the anatomical compartment surrounding blood vessels within the CNS.
The goal of this comprehensive review is to summarize the literature on perivascular spaces in human neuroinflammation and associated animal disease models. We describe the cell types taking part in the morphological and functional aspects of perivascular spaces and how those spaces can be visualized. Based on this, we propose a model of the cascade of events occurring during neuroinflammatory pathology. We also discuss current knowledge gaps and limitations of the available evidence. An improved understanding of perivascular spaces could advance our comprehension of the pathophysiology of neuroinflammation and open a new therapeutic window for neuroinflammatory diseases such as multiple sclerosis.
Link | PDF (Neuron) paywalled
Benjamin V. Ineichen; Serhat V. Okar; Steven T. Proulx; Britta Engelhardt; Hans Lassmann; Daniel S. Reich
It is uncontested that perivascular spaces play critical roles in maintaining homeostasis and priming neuroinflammation. However, despite more than a century of intense research on perivascular spaces, many open questions remain about the anatomical compartment surrounding blood vessels within the CNS.
The goal of this comprehensive review is to summarize the literature on perivascular spaces in human neuroinflammation and associated animal disease models. We describe the cell types taking part in the morphological and functional aspects of perivascular spaces and how those spaces can be visualized. Based on this, we propose a model of the cascade of events occurring during neuroinflammatory pathology. We also discuss current knowledge gaps and limitations of the available evidence. An improved understanding of perivascular spaces could advance our comprehension of the pathophysiology of neuroinflammation and open a new therapeutic window for neuroinflammatory diseases such as multiple sclerosis.
Link | PDF (Neuron) paywalled