The device stimulates the vagus nerve, signaling the body to tamp down the inflammation that contributes to the disease.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
New Implant Offers Hope for Easing Rheumatoid Arthritis
- Thread starter Jaybee00
- Start date
-
- Tags
- vagus nerve
Utsikt
Senior Member (Voting Rights)
Registration of the phase 3 study:
 clinicaltrials.gov
clinicaltrials.gov
Abstract:
 acrabstracts.org
acrabstracts.org
Maybe we should do a thread on the abstract?
ClinicalTrials.gov
Abstract:

Neuroimmune Modulation in Adults with Rheumatoid Arthritis and Inadequate Response or Intolerance to Biological or Targeted Synthetic DMARDs: Results at 12 and 24 Weeks from a Randomized, Sham-Controlled, Double-Blind Pivotal Study - ACR Meeting Abst
Background/Purpose: In this study, we evaluated the safety and efficacy of an implantable, cervical vagus nerve stimulation device for treatment of RA. Methods: This randomized, double-blind, sham-controlled study enrolled 242 patients at 41 sites in the U.S. with moderate to severe RA...
Maybe we should do a thread on the abstract?
Jonathan Edwards
Senior Member (Voting Rights)
Interesting that there was a lot of noise about this about 15 years ago and a large trial failed to show an effect. There appears to have been one more recent rial with an implanted device. I think the key question is whether blinding was reliable.
If this actually works and does not produce dangerous immunosuppression then everything we have learnt about immunology seems to have been a waste of time. A bit like the solution to your computer crashing being just to hit it with a hammer. So I am a wee bit sceptical.
If this actually works and does not produce dangerous immunosuppression then everything we have learnt about immunology seems to have been a waste of time. A bit like the solution to your computer crashing being just to hit it with a hammer. So I am a wee bit sceptical.
Neuroimmune Modulation in Adults with Rheumatoid Arthritis and Inadequate Response or Intolerance to Biological or Targeted Synthetic DMARDs: Results at 12 and 24 Weeks from a Randomized, Sham-Controlled, Double-Blind Pivotal Study
John Tesser, Joshua June, Pendleton Wickersham, Jane Box, Guillermo Valenzuela, Angela Crowley, Nikila Kumar, Norman Gaylis, Gordan Lam, David Ridley, Gineth Paola Pinto-Patarroyo, David Chernoff
[Line breaks added]
Background/Purpose
In this study, we evaluated the safety and efficacy of an implantable, cervical vagus nerve stimulation device for treatment of RA.
Methods
This randomized, double-blind, sham-controlled study enrolled 242 patients at 41 sites in the U.S. with moderate to severe RA, inadequate response or intolerance to at least 1 prior biological DMARD (bDMARD) or targeted-synthetic DMARD (tsDMARD). All patients remained on stable background conventional DMARDs, and were washed off their b/tsDMARD prior to implant procedure.
Randomization was 1:1 active (treatment) or non-active (control) stimulation. The primary endpoint was the proportion of patients achieving American College of Rheumatology 20% response (ACR20) at Week 12 from day of informed consent. Secondary endpoints included ACR20 from day of randomization, DAS28-CRP good/moderate EULAR and minimum clinically important difference (MCID) responses, and HAQ-DI response.
Exploratory endpoints included ACR50/70, DAS28-CRP low disease activity (LDA) and remission, and proportion of erosion progressors ( >0.5 progression) as measured by RAMRIS.
After Week 12, the study was open label, with one-way crossover of control to stimulation, with efficacy assessments repeated at Week 24. Patients were imputed as non-responder if rescued with steroids or b/tsDMARDs or if missing any data at Week 12.
Results
Baseline demographics and characteristics were well-balanced with mean age 56 years, 86% female, RA duration 12 years, BMI 30, 15 tender joints, and 10 swollen joints (28 joint count).
Number of prior b/tsDMARDs included 1 prior in 39%, 2 priors in 22%, and ≥3 priors in 39% of patients.
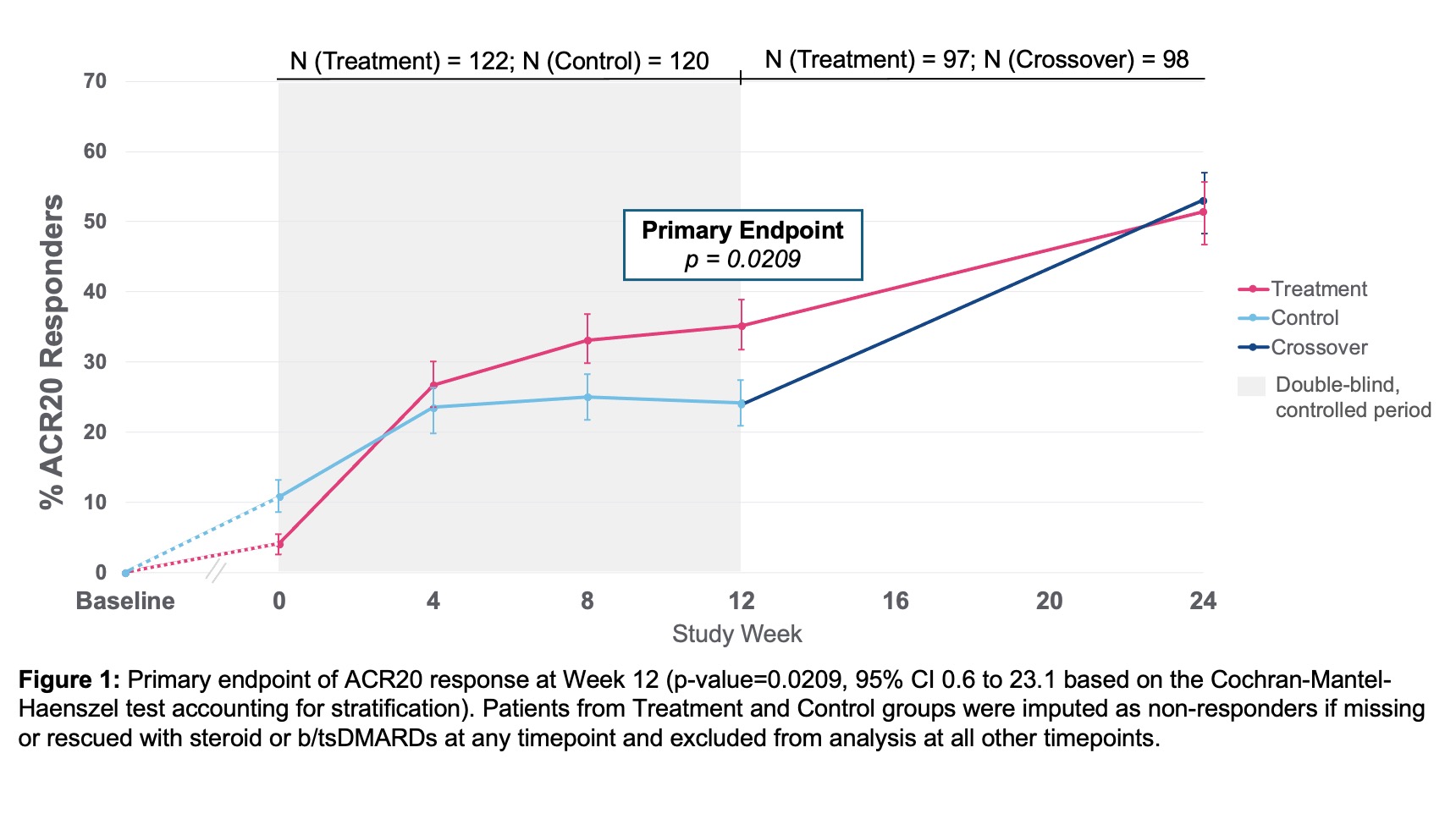
53% seropositive (RF and or anti-CCP) and mean hsCRP 8.2mg/L. ACR20 response at Week 12 showed a statistically significant difference between treatment 35.2% (43/122) vs. control 24.2% (29/120) (p-value=0.0209, 95% CI 0.6 to 23.1).
ACR20 response improved further to 51.5% in treatment and 53.1% in control, after crossover, by Week 24 (Figure 1). ACR20 response at Week 12 for patients with 1 prior bDMARD exposure (prespecified analysis) was treatment 44.2% (23/52) and control 19.0% (8/42) (p-value=0.0054, 95% CI 7.1 to 43.3).
All secondary and exploratory endpoints trended in favor of the treatment group.
DAS28-CRP LDA/remission at Week 12 showed a statistically significant difference between treatment and control groups and improved further through Week 24 (Figure 2). The proportion of erosion progressors was lower in the treatment vs. control group (Figure 3).
Overall, the safety profile was acceptable. Related serious adverse event (SAE)-rate was low (1.7%) by ITT. AE with highest frequency related to treatment was mild/moderate hoarseness/vocal cord dysfunction. Stimulation therapy specifically was well-tolerated. There were no deaths in the study.
At Week 24, 81% of patients were on stimulation therapy alone and free of added b/tsDMARD.
Conclusion
Neuroimmune modulation using an implantable device to stimulate the vagus nerve is well-tolerated and effective among adults with active RA disease and inadequate response or intolerance to at least one b/tsDMARD. (ClinicalTrials.gov, NCT04539964)
Web | Arthritis Rheumatol | Abstract only
John Tesser, Joshua June, Pendleton Wickersham, Jane Box, Guillermo Valenzuela, Angela Crowley, Nikila Kumar, Norman Gaylis, Gordan Lam, David Ridley, Gineth Paola Pinto-Patarroyo, David Chernoff
[Line breaks added]
Background/Purpose
In this study, we evaluated the safety and efficacy of an implantable, cervical vagus nerve stimulation device for treatment of RA.
Methods
This randomized, double-blind, sham-controlled study enrolled 242 patients at 41 sites in the U.S. with moderate to severe RA, inadequate response or intolerance to at least 1 prior biological DMARD (bDMARD) or targeted-synthetic DMARD (tsDMARD). All patients remained on stable background conventional DMARDs, and were washed off their b/tsDMARD prior to implant procedure.
Randomization was 1:1 active (treatment) or non-active (control) stimulation. The primary endpoint was the proportion of patients achieving American College of Rheumatology 20% response (ACR20) at Week 12 from day of informed consent. Secondary endpoints included ACR20 from day of randomization, DAS28-CRP good/moderate EULAR and minimum clinically important difference (MCID) responses, and HAQ-DI response.
Exploratory endpoints included ACR50/70, DAS28-CRP low disease activity (LDA) and remission, and proportion of erosion progressors ( >0.5 progression) as measured by RAMRIS.
After Week 12, the study was open label, with one-way crossover of control to stimulation, with efficacy assessments repeated at Week 24. Patients were imputed as non-responder if rescued with steroids or b/tsDMARDs or if missing any data at Week 12.
Results
Baseline demographics and characteristics were well-balanced with mean age 56 years, 86% female, RA duration 12 years, BMI 30, 15 tender joints, and 10 swollen joints (28 joint count).
Number of prior b/tsDMARDs included 1 prior in 39%, 2 priors in 22%, and ≥3 priors in 39% of patients.
53% seropositive (RF and or anti-CCP) and mean hsCRP 8.2mg/L. ACR20 response at Week 12 showed a statistically significant difference between treatment 35.2% (43/122) vs. control 24.2% (29/120) (p-value=0.0209, 95% CI 0.6 to 23.1).
ACR20 response improved further to 51.5% in treatment and 53.1% in control, after crossover, by Week 24 (Figure 1). ACR20 response at Week 12 for patients with 1 prior bDMARD exposure (prespecified analysis) was treatment 44.2% (23/52) and control 19.0% (8/42) (p-value=0.0054, 95% CI 7.1 to 43.3).
All secondary and exploratory endpoints trended in favor of the treatment group.
DAS28-CRP LDA/remission at Week 12 showed a statistically significant difference between treatment and control groups and improved further through Week 24 (Figure 2). The proportion of erosion progressors was lower in the treatment vs. control group (Figure 3).
Overall, the safety profile was acceptable. Related serious adverse event (SAE)-rate was low (1.7%) by ITT. AE with highest frequency related to treatment was mild/moderate hoarseness/vocal cord dysfunction. Stimulation therapy specifically was well-tolerated. There were no deaths in the study.
At Week 24, 81% of patients were on stimulation therapy alone and free of added b/tsDMARD.
Conclusion
Neuroimmune modulation using an implantable device to stimulate the vagus nerve is well-tolerated and effective among adults with active RA disease and inadequate response or intolerance to at least one b/tsDMARD. (ClinicalTrials.gov, NCT04539964)
Web | Arthritis Rheumatol | Abstract only
Jonathan Edwards
Senior Member (Voting Rights)
ACR20 response at Week 12 showed a statistically significant difference between treatment 35.2% (43/122) vs. control 24.2% (29/120) (p-value=0.0209, 95% CI 0.6 to 23.1).
That is an extremely feeble response. A decent agent shows a 40% or greater difference in CR20 rates, not 11%. I doubt most rheumatologists would consider it clinically significant. Useful responses are ACR50 or ACR70 anyway.
Utsikt
Senior Member (Voting Rights)
Secondary endpoints included ACR20 from day of randomization, DAS28-CRP good/moderate EULAR and minimum clinically important difference (MCID) responses, and HAQ-DI response.
They do not mention MCID in the results, only that the secondary outcomes trended in favour of the treatment group. The omission might indicate that the difference between the groups did not reach the threshold of MCID?All secondary and exploratory endpoints trended in favor of the treatment group.
hotblack
Senior Member (Voting Rights)
The trial seems to have been conducted by SetPoint who make the device, here’s their PR.
Are we thinking this is a complete waste of time or perhaps having a marginal effect somewhere, maybe downstream rather than directly modifying immunology?
What are the questions over the study…
Would the selection of only people who had an “inadequate response or intolerance to at least 1 prior biological DMARD” impact things?
The response does seem small, with questions over significance, but equally could even a small improvement be something for this group of people if there’s no significant negative effects? Would longer trials tell us anything?
It seems interesting they highlight a subgroup, presumably to strengthen their case, but I guess we need more detail on other subgroups and why they chose them?
Are we thinking this is a complete waste of time or perhaps having a marginal effect somewhere, maybe downstream rather than directly modifying immunology?
What are the questions over the study…
Would the selection of only people who had an “inadequate response or intolerance to at least 1 prior biological DMARD” impact things?
The response does seem small, with questions over significance, but equally could even a small improvement be something for this group of people if there’s no significant negative effects? Would longer trials tell us anything?
It seems interesting they highlight a subgroup, presumably to strengthen their case, but I guess we need more detail on other subgroups and why they chose them?
ACR20 response at Week 12 for patients with 1 prior bDMARD exposure (prespecified analysis) was treatment 44.2% (23/52) and control 19.0% (8/42) (p-value=0.0054, 95% CI 7.1 to 43.3).
Jonathan Edwards
Senior Member (Voting Rights)
I wouldn't waste time pondering this to be honest.



