NET Biomarkers in COVID-19 and Post-COVID Syndrome: a Comprehensive Analysis
SARS-CoV-2 triggers immune responses, including neutrophil activation and neutrophil extracellular trap (NET) release, contributing to COVID-19 severity. A significant proportion of patients experience persistent or new symptoms after recovery, referred to as post-COVID syndrome (PCS), which may be associated with NET release persistence. This study aimed to investigate whether NET-related biomarkers remain elevated after acute SARS-CoV-2 infection and to explore their association with PCS.
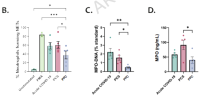
We conducted an analytical descriptive cohort study including patients with acute COVID-19 (n = 35), PCS (n = 35), and a pre-pandemic healthy control group (PPC; n = 35). Serum samples from participants were used to measure NET biomarkers, including MPO-DNA complexes, elastase-DNA complexes, and MPO levels. The capacity to induce NETosis in vitro was evaluated by incubating neutrophils with participant serum. Additionally, soluble immune markers, including cytokines, anti-SARS-CoV-2 antibodies, and autoantibodies, were assessed. A NETosis-based model was developed using NET biomarkers to distinguish between acute COVID-19 or PCS patients and PPC individuals.
Our findings revealed a significant increase in NET biomarkers during acute COVID-19 that persisted in PCS. This pattern was consistent with the in vitro study, where sera from PCS patients induced NET release. Additionally, NET biomarkers correlated with neutrophil counts, cytokines, autoantibodies, and inflammatory markers during acute COVID-19 and PCS. The multivariate model of NET biomarkers exhibited a high accuracy in distinguishing acute COVID-19 with an area under the curve (AUC) of 0.99 (CI = 0.98–1.00) and PCS patients with an AUC of 0.91 (CI = 0.84–0.99) from PPC.
These findings suggest that persistent NETosis may contribute to PCS pathophysiology, highlighting NETs as potential biomarkers and therapeutic targets in post-viral syndromes.
Web | DOI | PDF | Journal of Clinical Immunology | Open Access
Monsalve, Diana M; Numpaque-Morales, Laura; Rojas, Manuel; Acosta-Ampudia, Yeny; Ramírez-Santana, Carolina
SARS-CoV-2 triggers immune responses, including neutrophil activation and neutrophil extracellular trap (NET) release, contributing to COVID-19 severity. A significant proportion of patients experience persistent or new symptoms after recovery, referred to as post-COVID syndrome (PCS), which may be associated with NET release persistence. This study aimed to investigate whether NET-related biomarkers remain elevated after acute SARS-CoV-2 infection and to explore their association with PCS.
We conducted an analytical descriptive cohort study including patients with acute COVID-19 (n = 35), PCS (n = 35), and a pre-pandemic healthy control group (PPC; n = 35). Serum samples from participants were used to measure NET biomarkers, including MPO-DNA complexes, elastase-DNA complexes, and MPO levels. The capacity to induce NETosis in vitro was evaluated by incubating neutrophils with participant serum. Additionally, soluble immune markers, including cytokines, anti-SARS-CoV-2 antibodies, and autoantibodies, were assessed. A NETosis-based model was developed using NET biomarkers to distinguish between acute COVID-19 or PCS patients and PPC individuals.
Our findings revealed a significant increase in NET biomarkers during acute COVID-19 that persisted in PCS. This pattern was consistent with the in vitro study, where sera from PCS patients induced NET release. Additionally, NET biomarkers correlated with neutrophil counts, cytokines, autoantibodies, and inflammatory markers during acute COVID-19 and PCS. The multivariate model of NET biomarkers exhibited a high accuracy in distinguishing acute COVID-19 with an area under the curve (AUC) of 0.99 (CI = 0.98–1.00) and PCS patients with an AUC of 0.91 (CI = 0.84–0.99) from PPC.
These findings suggest that persistent NETosis may contribute to PCS pathophysiology, highlighting NETs as potential biomarkers and therapeutic targets in post-viral syndromes.
Web | DOI | PDF | Journal of Clinical Immunology | Open Access