A study with dual CD19-BCMA CAR-T, for 13 SLE patients is published, with up to 46 months of follow-up. All patients are in remission besides P11 who received half of the dose (see the quote below)
It seems they have used much less chemotherapy,they mention only 300mg/m2, cyclophosphamide, and only the first 2 patients received any flu (there was no additional benefit it seems). So 3 x less chemo? or they've omitted to write 300 mg x 3 days?
Also, interesting (and scary) part:
Strange that even dsDNA came back, which never happened from what I know with German patients treated with only CD19, even.
Makes sense that the dual needs higher dose, though? Especially if chemo is 3x lower as well
.
Also, it's interesting that they measured IgA levels in saliva, which seem to come back at 8 months (not yet normal for everyone), and they did renal biopsy:
It seems they have used much less chemotherapy,they mention only 300mg/m2, cyclophosphamide, and only the first 2 patients received any flu (there was no additional benefit it seems). So 3 x less chemo? or they've omitted to write 300 mg x 3 days?
Patient treatment
T cells from peripheral blood obtained via apheresis were transfected to engineer cCAR. Treatment schema is depicted in figure 1A. Patients received a dose of 3×10^6 cCAR cells /kg body weight postcessation of all SLE medications following
conditioning with cyclophosphamide (0.3g per m2 ) and fludarabine (0.03g per m2 ) (Cy/influenza) for P1 and P2, and Cy (0.3g per m2 ) alone for P3–P13. SLE/LN patients receiving immunosuppressing medications may suffer severe lymphopenia
due to conditioning and risk subsequent infection. Given that Cy conditioning has demonstrated comparable efficacy to Cy/influenza, Cy alone was trialled to mitigate this possibility.12 The trial commenced as of 17 September 2019 and 13 patients have received cCAR as of 1 October 2023.
Also, interesting (and scary) part:
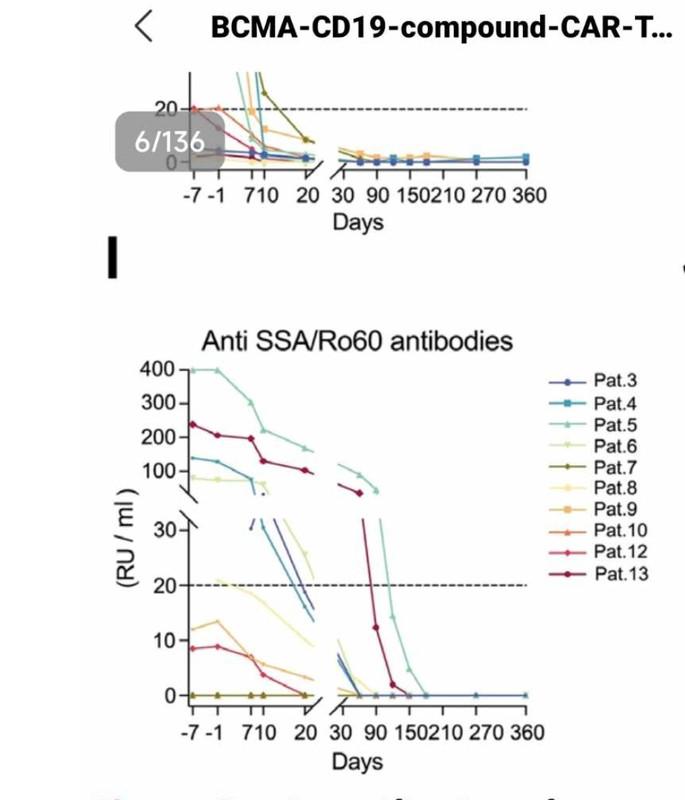
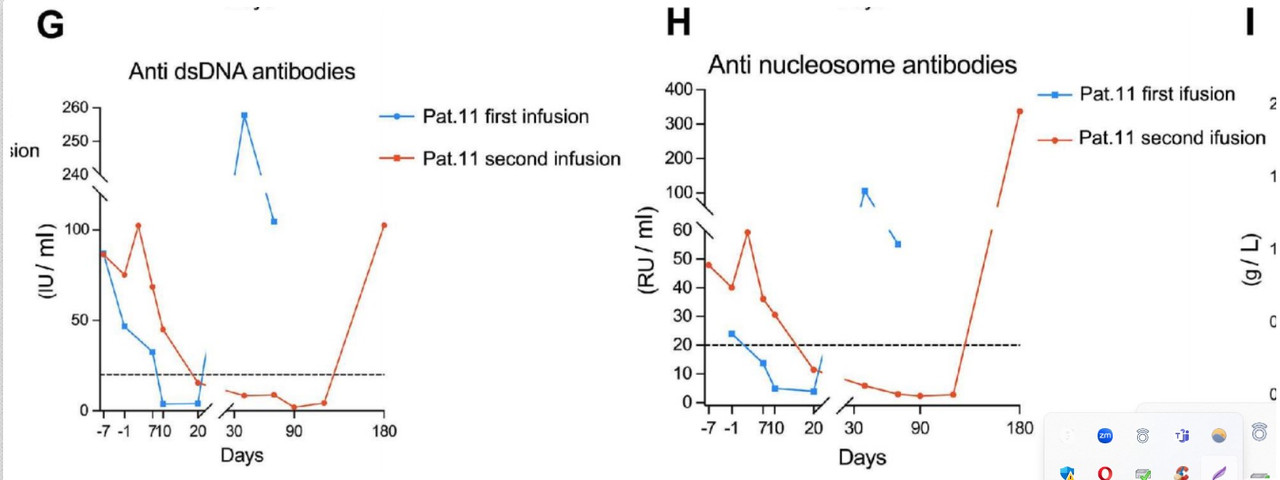
Severe bone marrow suppression was experienced by P11 and therefore inadequate T cell counts were harvested through apheresis. As compassion-focused therapy, P11 received a dose of 1.5×10°cells /kg. This initial dose given to P11 proved to be subtherapeutic, even so, P11 still achieved medication and symptom-free LLDAS for 4 months. Unfortunately, clinically insufficient autoantibody control was observed which indicates that the dose of the initial infusion of CAR T cells is critical. Relapse was observed 4 months following the initial cCAR infusion and as such P11 was reinfused with the target dose. Despite this subsequent dosing (3×10°cells/kg), P11 relapsed 6 months following treatment. Similar relapse has been observed with CD19 CAR T cells, where redosing may result in failure rates as high as 80%. The initial relapse was a result of insufficient dosing, however, the subsequent relapse following reinfusion was likely a result of anti-CAR antibodies. This instance demonstrates the critical importance of a sufficient initial dose. Completing the second reinfusion in closer proximity to the initial dose, or an increased reinfusion dose may be other mechanisms for solving this problem. Strong persistence of cCAR was observed as symptom and MFR were maintained following reinfusion for 6 months despite the presence of anti-CAR neutralising antibodies.
After the initial dose of 1.5×106 cCAR cells /kg, P11 achieved
symptom-free and MFR that persisted for 4months. Relapse was
observed, particularly in control of autoantibodies which appeared
at 6–8 weeks, indicated by an increase in anti-U1-snRNP, dsDNA,
anti-Sm and anti-nucleosome
Strange that even dsDNA came back, which never happened from what I know with German patients treated with only CD19, even.
Makes sense that the dual needs higher dose, though? Especially if chemo is 3x lower as well
.
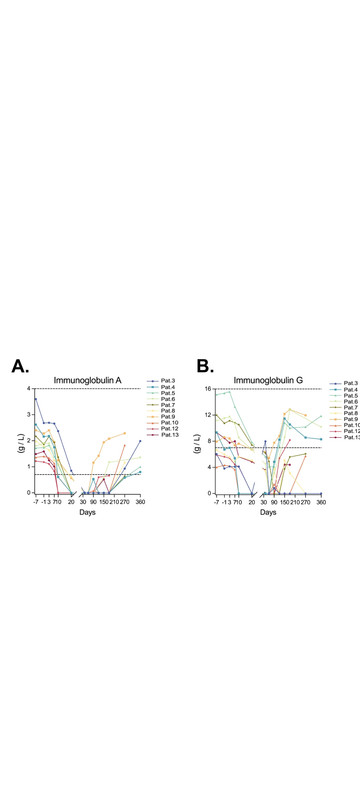
Also, it's interesting that they measured IgA levels in saliva, which seem to come back at 8 months (not yet normal for everyone), and they did renal biopsy:
Immunofluorescence analysis of renal biopsy. The pathology in P3 illustrates the deposition of immunoglobulin IgA, IgM, IgG, complement C3, C1q, and fibrin (FIB) in the kidneys before cCAR and 6 months after cCAR treatment.
As can be seen above, following cCAR treatment, except for a small quantity of IgM deposition in the renal tissue, all other glomerular insults were significantly reduced or absent.
Last edited: