Metabolic Disorders Causing Fatigue in Sjogren’s Syndrome
ABSTRACT NUMBER: 2546
Metabolic Disorders Causing Fatigue in Sjogren’s Syndrome
Lakshmanan Suresh1, Julian Ambrus2, Long Shen3 and Sahana Vishwanath4, 160 Pineview Drive, State University of New York/Buffalo, Buffalo, NY, 2100 High St., University Of Buffalo, Buffalo, NY, 3Department of Medicine, SUNY at Buffalo, Buffalo, NY, 4Medicine, SUNY- Buffalo, buffalo, NY
Meeting: 2014 ACR/ARHP Annual Meeting
SESSION INFORMATION
Title: Sjogren's Syndrome: Clinical Science
Session Type: Abstract Submissions (ACR)
Background/Purpose
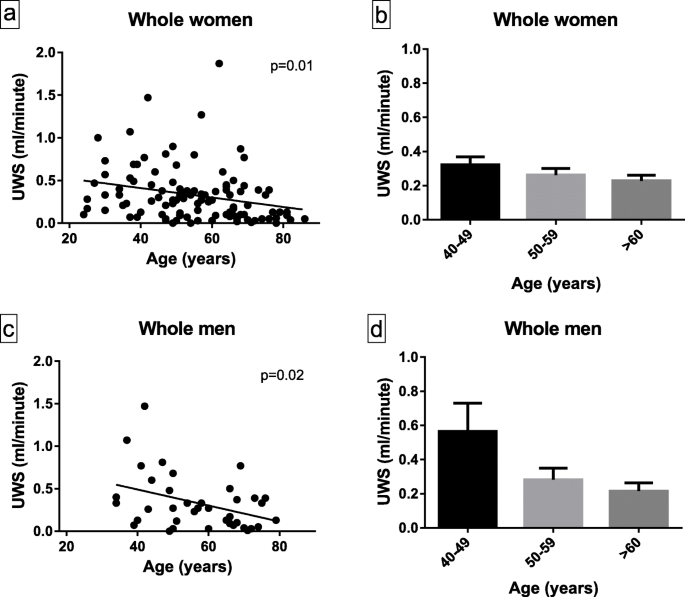
Sjogren’s syndrome (SS) is a complex disorder involving both the innate and immune system. Fatigue is a common feature of the disease. Mitochondrial dysfunction has been associated with chronic inflammatory diseases, including SLE, and can result in fatigue and exercise intolerance.
Methods
We evaluated 32 patients meeting the ACR criteria for SS who presented with profound fatigue for disorders of aerobic and anaerobic metabolism. Serum lactate, carnitine and ischemic forearm tests were performed. In selected patients, muscle biopsies were done for genetic and biochemical studies.
Results
Of these SS patients, 30 had elevated lactic acid at rest and 3 had abnormal ischemic forearm tests. Nine of the patients underwent muscle biopsies. Four had mitochondrial respiratory chain abnormalities, 2 had carnitine palmitoyl transferase deficiency, 1 had very long chain acyl CoA dehydrogenase deficiency and 2 had glycogen storage diseases (myophosphorylase deficiency, lactate dehydrogenase deficiency). Appropriate treatment led to symptomatic improvement in all cases.
Conclusion
Metabolic disorders are common in patients with SS and contribute to symptoms such as fatigue and exercise intolerance. Treatment of these disorders leads to symptomatic improvement. Further studies are needed to determine the incidence of metabolic disorders in SS, the incidence of SS in patients with metabolic disorders and the mechanisms by which SS and metabolic disorders are interrelated.
ABSTRACT NUMBER: 2546
Metabolic Disorders Causing Fatigue in Sjogren’s Syndrome
Lakshmanan Suresh1, Julian Ambrus2, Long Shen3 and Sahana Vishwanath4, 160 Pineview Drive, State University of New York/Buffalo, Buffalo, NY, 2100 High St., University Of Buffalo, Buffalo, NY, 3Department of Medicine, SUNY at Buffalo, Buffalo, NY, 4Medicine, SUNY- Buffalo, buffalo, NY
Meeting: 2014 ACR/ARHP Annual Meeting
SESSION INFORMATION
Title: Sjogren's Syndrome: Clinical Science
Session Type: Abstract Submissions (ACR)
Background/Purpose
Sjogren’s syndrome (SS) is a complex disorder involving both the innate and immune system. Fatigue is a common feature of the disease. Mitochondrial dysfunction has been associated with chronic inflammatory diseases, including SLE, and can result in fatigue and exercise intolerance.
Methods
We evaluated 32 patients meeting the ACR criteria for SS who presented with profound fatigue for disorders of aerobic and anaerobic metabolism. Serum lactate, carnitine and ischemic forearm tests were performed. In selected patients, muscle biopsies were done for genetic and biochemical studies.
Results
Of these SS patients, 30 had elevated lactic acid at rest and 3 had abnormal ischemic forearm tests. Nine of the patients underwent muscle biopsies. Four had mitochondrial respiratory chain abnormalities, 2 had carnitine palmitoyl transferase deficiency, 1 had very long chain acyl CoA dehydrogenase deficiency and 2 had glycogen storage diseases (myophosphorylase deficiency, lactate dehydrogenase deficiency). Appropriate treatment led to symptomatic improvement in all cases.
Conclusion
Metabolic disorders are common in patients with SS and contribute to symptoms such as fatigue and exercise intolerance. Treatment of these disorders leads to symptomatic improvement. Further studies are needed to determine the incidence of metabolic disorders in SS, the incidence of SS in patients with metabolic disorders and the mechanisms by which SS and metabolic disorders are interrelated.