siobhanfirestone
Senior Member (Voting Rights)
Thanks, @Hutan . You outlined the reasons why I think the data are still relevant really well – you saved me a post!
I think what’s difficult, @siobhanfirestone , is that you’re posting your story – which is fabulous; on a personal level it is so wonderful to hear of someone going from sick to apparently healthy and I and others are delighted for you – on a science forum, so people are responding in sciencey ways. I think all that cautious people are saying on here is that we are not comfortable saying anyone's remission is likely due to cyclo until a randomised, placebo-controlled trial is positive.
I think it’s worth looking at the last few publications from Rekeland et al. – i.e. the Norwegian Fluge and Mella group.
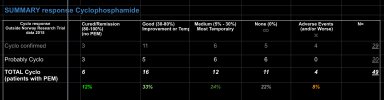
In 2020, Rekeland et al. published the results of the cyclophosphamide study. https://pubmed.ncbi.nlm.nih.gov/32411717/ They concluded:
Another paper by the same group, Rekeland et al. 2022, is also very interesting in this regard. This was not a treatment study. They just followed people with ME/CFS for 6 months using Fitbits and questionnaires. Their data are publicly available. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9484698/
Out of 14 patients who were moderate at baseline (SF36PF 30-59 in weeks 1-4):
- 3 reported SF36PF scores that were ≥20 points higher in the final month (weeks 21-24). The biggest jump was 45 points.
- The step count of 4 patients (i.e. 29%) increased by more than 2000 steps in this time period.
- One patient who started at 3900 steps per day finished at 10219 steps per day.
- Another started at 4453 and finished at 8661, all in 6 months of no intervention.
They conclude:
Then Rekeland et al. 2024 present promising data from a 6 year follow-up, and conclude https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11265720/:
What people are doing on here is just heeding the warnings of these excellent scientists, and learning from long experience of our own remissions and relapses, improvements and deteriorations, treatments tried, and the rituximab rollercoaster.
Edited formatting
Oh i wouldnt expect these doctors to do anything else, but I chose myself what I wanted. Thats not going to be something everybody can and should do, and I have purposfully with-held information so that its impossible to know how I went about this, this is not me saying that this drug is key - its me saying my own case study indicates IgG reduction is what caused remission during chemo.