Dear all,
Here is the latest update. cc
@wigglethemouse ,
@Perrier @Ben H ,
@rvallee ,
@ScottTriGuy
I believe that significant progress has been made ( meaning for symptoms related to myself ,so n=1 !). In order to better understand how is all connected i've been crashing myself purposely to perform root cause analysis.
For this reason - with a little help from technology - i am trying to identify how different promising research targets (hypoperfusion, sepsis, inflammatory response,hypoxia, glutamate and others etc) fit the picture. I would like to provide a small example of the work i've been doing. Please see below :
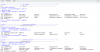
The map shows a learned network of interactions between these topics using machine learning(the best way to put it). Observe how inflammatory response is connected with sepsis and the gut / albumin. Why the connection with albumin ? Perhaps because inflammation lowers albumin levels. My first question is : D
o we find hypoalbuminemia or even hyperalbuminemia to ME patients ?
We next see the connection with "ckd" which is chronic kidney disease, a topic which i have mentioned many times. Here is where eGFR may be coming to the picture.
Do ME patients are found with significantly lower eGFR than Healthy controls? We move through ckd to angiotensin, endothelin to vasoconstriction which leads (?) to hypoxia and hypoperfusion (?).
Please note, this is just an example of the tools that we already have and no one is currently using. These tools are supposed to work with the help and guidance of medical professionals.
I
hypothesise the following for further research :
a) The Inflammatory response is a key piece of the puzzle. Proposed topics here are IL-6 and TNF-α
b) The same applies for glutamate/kynurenine metabolism. The common factor of these two is excitotoxicity and tinnitus may be related to vasocontriction / hypoxia/ excitotoxicity or even hypoperfusion
c) Metabolism of Nitric oxide : more work is needed here
I can induce the perfect storm if i eat too much protein (whey protein has a negative effect too), Parmesan cheese (contains glutamate) and many other glutamate sources. Please have a look here :
https://www.msgtruth.org/what-foods-should-i-avoid
and also the "Umami" database :
https://www.umamiinfo.com/umamidb/search/
I cannot really suggest you try a glutamate-full diet because it may induce a severe crash (if the theory is applicable). Whatever i write here is
not a suggestion to try but for informational purposes only.
I move on to -yet- another available tool that is not currently being used to speed things up. I will provide an example based on the above. As discussed, inflammatory response may be a key topic related to ME pathology. Imagine being a researcher and you wish to see which compounds have an effect on ameliorating the inflammatory response. At the moment of writing, "inflammatory response" has 72479 entries on PUBMED. Using an Information Extraction tool, we are getting the following excerpts which contain (almost) exactly what we need.
**************************************************** : Improvement**********************************************
. Magnesium sulfate (MgSO4) has been known to ameliorate maternal, fetal and gestational tissue-associated inflammatory response.
**************************************************** : Improvement**********************************************
. Resveratrol significantly improved kidney function and lowered serum and kidney tissue inflammatory cytokine levels. Consistently, resveratrol prevented endotoxin-induced disruption of endothelial cell permeability and inhibited inflammation of kidney tissue.
**************************************************** : Improvement**********************************************
. In these patients N-acetyl cysteine and vitamin administration can be considered as an effective method for improvement of oxidative status.
**************************************************** : Improvement**********************************************
. CONCLUSIONS: IAP is a major regulator of gut mucosal permeability and is able to ameliorate starvation-induced gut barrier dysfunction. Enteral IAP supplementation may represent a novel approach to maintain bowel integrity in critically ill patients.
**************************************************** : Improvement**********************************************
, patients improved after therapy with high-dose thiamine.
**************************************************** : Improvement**********************************************
. Oral or parenteral therapy with high-dose thiamine was started. RESULTS: The therapy led to an appreciable improvement of fatigue.
**************************************************** : Improvement**********************************************
. Inhibition of arginase with an arginase-specific inhibitor, N(omega)-hydroxy-nor-L-arginine, ameliorates the DNCB-induced inflammatory response. Our results demonstrate that HPV16.
**************************************************** : Improvement**********************************************
. From these results, we conclude that supplementation of EGCG improves glucose tolerance, insulin sensitivity, and endothelial function.
**************************************************** : Improvement**********************************************
. CONCLUSION: These findings suggest that treating infants undergoing CPB with a lipid emulsion containing omega-3 improves fatty acid status and results in a lower inflammatory response after surgery.
**************************************************** : Improvement**********************************************
. The restoration of their levels by either exogenous administration of these mediators or feeding omega-3-enriched diets, improves the inflammatory status of adipose tissue and ameliorates metabolic dysfunction.
**************************************************** : Improvement**********************************************
. However, the human data are inconclusive as to whether omega-3 PUFA supplementation at this dosage is effective in attenuating the inflammatory and immunomodulatory response to exercise and improving exercise performance.
**************************************************** : Improvement**********************************************
. Acetyl-L-carnitine treatment decreases the severity of mental and physical fatigue, depression cognitive impairment and improves health-related quality of life.
The list above is not the complete output.
The extraction of this information from 72479 PUBMED abstracts required a total runtime of 1 minute and 24 seconds.