mariovitali
Senior Member (Voting Rights)
@Ravn
I believe that HRV is a very important predictor so bought a heart monitor strap and connected it to my phone. I got my first reading (see attached) which is considered a low reading. I also stared a drug which was proposed through Machine Learning . I know all the signs that my body gives and so i will know within a few days. I expect also that the HRV will increase over the next period of time.
In any case this is really exciting, i will post updates.
@Trish @Andy do you think that HRV monitoring deserves a different thread?
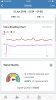
I believe that HRV is a very important predictor so bought a heart monitor strap and connected it to my phone. I got my first reading (see attached) which is considered a low reading. I also stared a drug which was proposed through Machine Learning . I know all the signs that my body gives and so i will know within a few days. I expect also that the HRV will increase over the next period of time.
In any case this is really exciting, i will post updates.
@Trish @Andy do you think that HRV monitoring deserves a different thread?

Last edited:



