- Home
- Forums
- Discussion topics
- Causes, Epidemiology, Criteria, Naming discussions
- Possible causes and predisposing factor discussion
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Long-lived plasma cell (LLPC) theory - Similarities between CFS and Lupus?
- Thread starter ryanc97
- Start date
ryanc97
Senior Member (Voting Rights)
I never said a single dose is needed. I don't claim to know about Lupus mechanism. But it has been used@ryanc97 are you suggesting a single dose of Daratumab in Lupus leads to permanent disease halt without the need for a maintenance therapy? Is there data on that?
Daratumumab monotherapy for refractory lupus nephritis - Nature Medicine
In a case series of six patients with refractory lupus nephritis who were treated off-label with an anti-CD38 monoclonal antibody, five patients achieved complete or partial renal responses by 12 months of follow-up.
But if you don't know this then the comparison fails. If one was to believe the remissions in ME/CFS were drug related (for which there is of course no evidence) then they were permanent and there was no need for maintenance dosages, if this in not the case in Lupus then things have to be different. Which as all what I've said. Does that make things clearer?I never said a single dose is needed. I don't claim to know about Lupus mechanism. But it has been used
Kitty
Senior Member (Voting Rights)
Hence any theory must account for this.
That's hardly difficult, though. Placebo effects and natural changes in the level and severity of ME/CFS symptoms are really common.
This is just a theory. I've seen dozens of them over the years; they were all superficially convincing and they were all wrong. I'll be as happy as anyone else if it turns out to be right, but I'm not putting any money on it.
I'd be surprised if even the team involved really believes they've got the final answer. They're just pursuing a research question that will hopefully tell us something useful.
According to Google there are also responses with Rituximab in Lupus for 12 months, but google also says that it's suggested that "memory isn't removed in general". The study you cited above only had a 12 month follow-up so I'm not sure whether it is relevant to the discussion on whether "memory was removed or not" because 12 months doesn't seem permanent.I never said a single dose is needed. I don't claim to know about Lupus mechanism. But it has been used

Daratumumab monotherapy for refractory lupus nephritis - Nature Medicine
In a case series of six patients with refractory lupus nephritis who were treated off-label with an anti-CD38 monoclonal antibody, five patients achieved complete or partial renal responses by 12 months of follow-up.www.nature.com
I don't think you're understanding what I'm trying to say so I'll leave the discussion now.
ryanc97
Senior Member (Voting Rights)
@Jonathan Edwards how many other drugs have had the same level of rigour of a trial like Rituximab did for CFS? Is Rituximab the only one?
Jonathan Edwards
Senior Member (Voting Rights)
Where did you find individual time series data of the patients in P2? I can't find it in the paper.
Also I disagree, I think that some patients had remission on Ritux means something is going on.
Also, if Ritux is said to not work, that suits the theory fine because you can say Rituximab doesn't touch LLPCs. Or at least the 20% of LLPCs with CD20 are not faulty.
And also, if P3 is definitive to say whether something works or not, how many drugs have a P3 for CFS?
Oystein Fluge sent me all the raw time course data personally because we were discussing the case for further studies together.
I can assure you that, as the guy who started all this ritux business for non-haematologic disease and someone who has been involved in trials of all sots of things over the years that people having remissions while on trials does not in any way mean 'something is going on' in the sense of the drug being responsible. People often get better during trial periods. Lots of people in the placebo arm of the Norwegians' trials got completely or nearly completely better. I used the Norwegian phase 2 data when reporting to NICE as a particularly vivid example of how you cannot assume that improvement is due to treatment.
I agree that if the theory is that bad antibodies are being made by LLPC then a nul result with rituximab fits. But the cyclo response only fits if there was significant fall in Ig levels. Normally you would need high doses of cyclo to achieve a fall.
ryanc97
Senior Member (Voting Rights)
Is there a reason why they don't publish the individual data in P2 or P3? Not publishing it seems quite convenient if you have people achieving remission on pure placebo..... why wouldn't you want to show that?Oystein Fluge sent me all the raw time course data personally because we were discussing the case for further studies together.
I can assure you that, as the guy who started all this ritux business for non-haematologic disease and someone who has been involved in trials of all sots of things over the years that people having remissions while on trials does not in any way mean 'something is going on' in the sense of the drug being responsible. People often get better during trial periods. Lots of people in the placebo arm of the Norwegians' trials got completely or nearly completely better. I used the Norwegian phase 2 data when reporting to NICE as a particularly vivid example of how you cannot assume that improvement is due to treatment.
I agree that if the theory is that bad antibodies are being made by LLPC then a nul result with rituximab fits. But the cyclo response only fits if there was significant fall in Ig levels. Normally you would need high doses of cyclo to achieve a fall.
Jonathan Edwards
Senior Member (Voting Rights)
@Jonathan Edwards how many other drugs have had the same level of rigour of a trial like Rituximab did for CFS? Is Rituximab the only one?
It is the one that comes to mind. But there have been trials of anti-virals with negative outcomes too.
And also, if P3 is definitive to say whether something works or not, how many drugs have a P3 for CFS?
P3 tends to be definitive for showing that something really does work. But most of the time P2 is enough to show that no useful effect is observed. Phase 3 is there to ensure that positive data are good enough to be relied on. Pretty much everything else for ME/CFS has failed by being unconvincing in early studies. Even if those studies were reported as showing some promise and justifying further trials the absence of further trials tends to indicate that nobody has much confidence in that. Fluge and Mella went to phase 3 despite a technical negative at phase 2 because they were committed to getting a clear answer. They did a great job but the final answer was again negative.
Jonathan Edwards
Senior Member (Voting Rights)
Is there a reason why they don't publish the individual data in P3? Not publishing it seems quite convenient if you have people achieving remission on pure placebo..... why wouldn't you want to show that?
I was talking about detailed time course profiles, which involve huge amounts of data. You don't normally publish those. I think the primary outcomes were published for phase 3 and you can see how many people had major improvements. I have not looked at that in detail. I used the phase 2 data to make the point with NICE.
Did cyclophosphamide treatment reduce Ig levels?
Yes cyclo did reduce Ig levels. Please see my post here
Post in thread 'Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Clinical trials, medical treatment and pathomechanisms, 2024, Rekeland'
https://www.s4me.info/threads/myalg...thomechanisms-2024-rekeland.38262/post-549662
ETA fixed wrong link.
OK in my haste I got this wrong. The non-responders were in blue—my bad. So please ignore the thing I wrote below. But it is the reverse of the dara trial—in the cyclo study high IGG4 were non-responders.
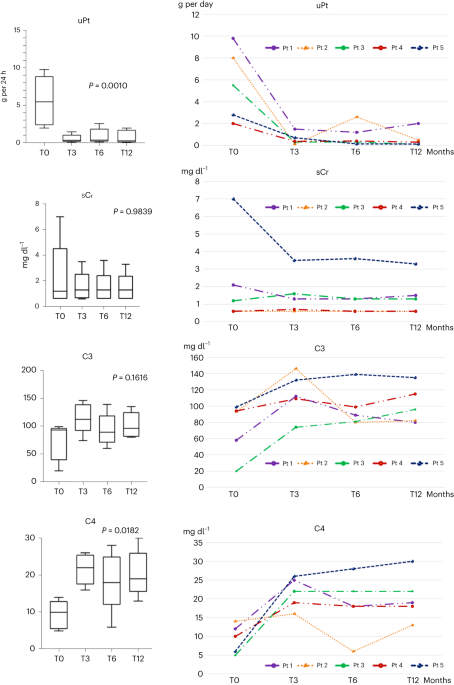
Wait a minute!!! If you look at the cyclo data for IGG4 we see the same phenomenon as occurred in the Daratumumab trial. Low IGG4 patients were non-responders!!! Fig. C.
Post in thread 'Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Clinical trials, medical treatment and pathomechanisms, 2024, Rekeland'
https://www.s4me.info/threads/myalg...thomechanisms-2024-rekeland.38262/post-549662
Post in thread 'Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Clinical trials, medical treatment and pathomechanisms, 2024, Rekeland'
https://www.s4me.info/threads/myalg...thomechanisms-2024-rekeland.38262/post-549662
Last edited:
ryanc97
Senior Member (Voting Rights)
You might have flipped the groups there mateWait a minute!!! If you look at the cyclo data for IGG4 we see the same phenomenon as occurred in the Daratumumab trial. Low IGG4 patients were non-responders!!! Fig. C.
Post in thread 'Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Clinical trials, medical treatment and pathomechanisms, 2024, Rekeland'
https://www.s4me.info/threads/myalg...thomechanisms-2024-rekeland.38262/post-549662
Oops you are right… my bad. I corrected.You might have flipped the groups there mate
Last edited:
ryanc97
Senior Member (Voting Rights)
@Jonathan Edwards, wanted to ask, from reading Lupus studies and their subreddit for Dara/Cyclo/Ritux, it seems like they require continued dosage of the drug in order to have improvements? Aka continued immunsuppression?
For example a commenter says for Lupus "I have been on Rituximab for 5 years now".
Which seems to imply that the faulty antibodies production cycle starts again if you stop?
Which then implies the fault is very upstream/far back in the pipeline like at the stem cell/B cell level? Is this is a correct understanding?
For example a commenter says for Lupus "I have been on Rituximab for 5 years now".
Which seems to imply that the faulty antibodies production cycle starts again if you stop?
Which then implies the fault is very upstream/far back in the pipeline like at the stem cell/B cell level? Is this is a correct understanding?
Last edited:
ryanc97
Senior Member (Voting Rights)
According to Google there are also responses with Rituximab in Lupus for 12 months, but google also says that it's suggested that "memory isn't removed in general". The study you cited above only had a 12 month follow-up so I'm not sure whether it is relevant to the discussion on whether "memory was removed or not" because 12 months doesn't seem permanent.
I don't think you're understanding what I'm trying to say so I'll leave the discussion now.
Yes it seems to me that in Lupus the symptons are less severe than CFS, but apparently there is memory, like you reset the B cells / plasma cells, but after you clear them out, the same faulty Lupus-AAB producing cells come back again.
It would seem the leak for Lupus is very much far upstream/closer to source (aka the stem cells) than CFS?
Last edited:
Arfmeister
Senior Member (Voting Rights)
There is actually Cyclo data on IgG levels.For some reason, the paper 2015 cyclo trial does not show IGG levels at all.
Cyclo responders had the lowest IgG drop - on average
But not a drop to the extent of the n=10 Dara pilot.
Also, the starting IgG from responders was already lower than non-responders (on average)
EDIT: @Jaybee00 i missed your comment (️)
So this is a double entry.

Attachments
Last edited:
Arfmeister
Senior Member (Voting Rights)
Mella is speculating on what might explain things, but the absence of any difference in the phase 3 trial makes it much most likely that it simply does not work.
Why can’t we state that Ritux might have worked for a subgroup?
Drug trials are hold to the highest standards, rightly so, as we need scientific rigor.
But it doesn’t leave room for the middle ground: Either it is placebo, or the drug is effective as it’s theorized.
- so IF it doesn’t work according to the pre-set objectives and targets - with the theorized mechanism of action
- it immediately is defined as placebo = patients are solely in remission because of psychological effect.
(Or they are the relapse remitting type (?)
BUT it might still be possible that Ritux works for a subgroup by an unknown bio-mechanism.
I know 2 long-time very severe patients that got into remission by Ritux.
- they were definitely not the relapse remitting ME-patient, more the progressive long-term severe
- now we can not discard placebo, but for me, knowing the 2 case studies, Ritux must have had an (yet unknown) effect in their remission
F & Mella are also of the opinion that some of the Ritux remissions are real and not to be ignored.
– and they know these patients (case studies) from very close by with all the detail
PS: and isn’t it common in many autoimmune diseases (for e.g. Rheuma. A. or M.S.) that :
- certain drugs work for a small % of patients, and others drugs work for another group, and some patients don’t respond at all ?
PS2: the fact that a big % of ME patients is of the relapse-remitting type is tainting every drug trial that is done.
We need to take this into account.
Last edited:
Because you have to establish efficacy on a population level basis first. For the most part, all the drugs that work have been shown to work in phase 3 studies, even when they don't work for everybody (or there is a reasoning why they should work a sensible dose-response curve or something else). That applies to all the drugs that you mention above even if they don't always work. If there are subgroups, all you need is sufficient sample sizes and the Phase 3 sample size of the Rituximab was large enough to see if there are benefits outweigh the risks. All you need is large enough sample sizes.Why can’t we state that it might work for a subgroup?
Isn’t it the same for e.g. Rheuma. A. or M.S. ?
- certain drugs work for some groups, others work drugs work for another group and some patients don’t respond at all ?
If you argue that the drug works for a subgroup, you necessarily also have to argue that placebo works for a subgroup because as many people in the placebo group were responders to the trial. Both groups look the same and the amount of people that got better are roughly equal. Since the placebo is a lower risk and risks tend to increase outside of trials the argument would then be: Give placebo to people with ME/CFS. I think that quickly shows how ridiculous the arguments about subgroups become. Of course subgroups responding to Rituximab theoretically might exist but you cannot argue that the trial provides evidence of this because it doesn't, it only provides evidence of no efficacy of the drug in treating ME/CFS (reported improvement might even more likely be due to placebo, regression to mean, Hawthorne effect, non-objective outcomes, natural remission etc etc etc). Effects like regression to mean, Hawthorne effect, natural remission ect aren't "all in the mind" and don't suggest that ME/CFS is a BPS problem, these effects are witnessed across conditions that aren't "all in the mind".
If you say "I cannot believe these effects were not due to the drug because I think that would mean ME/CFS is BPS problem", you are actually automatically saying "ME/CFS is a BPS problem" because just as many people got better in the placebo group!
ME/CFS and Rituximab are not special. You're argument also applies to CBT/GET and even scuba diving for ME/CFS and any other bogus intervention that exists for any conditon.
Last edited:

