First part:
From: Jo Røislien
Sent: Wednesday 16th of november 2022 10:00
To: Camilla Bø Iversen
Hi Camilla,
I'm sending the SMS and email as I mentioned yesterday. Below is the SMS I received from Signe Flottorp 31. march 2022 at 14:40, and my reply 17:05 the same day. I then received and email from her (that I will send separately). This email I did not reply to.
Jo
(This is the SMS from Flottorp)
Hi Jo. Hope all is well. If you have time for a chat that would be nice. It's about Live Landmarks LP study. On another note I can send my regards from ----, and I came here saturday and will stay until after easter. We have started olive harvesting a few years ago and will now cut the trees. We are enjoying ourselves. Regards, Signe
The separate email:
From: Signe Flottorp
Sent: Tuesday 5th of april 2022 16:47
To: Jo Røislien
Topic: The ME-debate
Ho Jo, sorry for the slow response, I'm in vacation mode.
Here is an email - it's a bit long

I've been involved in the ME debate in Norway since Kunnskapssenteret (Knowledge center) produced the first systematic review of treatment of CFS/ME in 2006 with an interdisciplinary group of Norwegian clinicians and researchers, and with user involvement. Vegar B Wyller (sic) was first author, Lillebeth Larun lead the work, and I was project manager. The ME Association protested against the report when it was published. Later colleagues at the Kunnskapssenteret has produced several systematic reviews related to CFS/ME. I've been involved in some of them, among others the review of a Cochrance review about the effect of exercise for patients with CFS/ME (Lillebeth Larun at FHI was first author), after pressure from ME-activists from the UK, who in the first round had managed to persuade the editors at Cochrane to withdraw the review. We stopped this after a solid factual review, in a pretty tough discourse between FHI and Cochrane. We were aware that the ME activists in the UK wanted to make Cochrane withdraw the review due to the then ongoing process of upgrading the NICE guidelines for CFS/ME in the UK. NICE changed previous guidelines pretty radically by no longer advising the use of exercise and cognitive behaviour therapy, the only evidenced treatments. NICE warns against LP explicitly, despite for the only RCT that has been conducted showing positive effects.
I submitted a comment to the draft NICE guidelines from FHI together with colleague Per Magnus and Vegard BB Wyller in dec. 2020 (see attachment).
A row of critical submissions from professional bodies in the UK and others did not cause major changes to the final version.
Lancet incited me to write a comment on the NICE guidelines - I wrote together with Vegard BB Wyller, Kjetil Brurberg (colleague at FHI) and colleagues from Netherland and Denmark (see attachment).
I am in contact with multiple clinicians and researchers that work with research to increase our understanding of CFS/ME (and now also long term sequale of covid-19). The dichotomy physical/psychological is most unfortunate - it should be elementary that the head is part of the body. Here is an illustration of a model that illustrate how biological, psychological and social factors can contribute to the different symptoms one can experience following a viral infection:
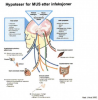
Symptoms can be seen as homeostatic alarms (useful during acute infection) that activates stress responses. Symptoms can chronify by the alarms getting "stuck".
Recovery Norway convey strong stories about people who have recovered from CFS/ME and similar illnesses, among other ways by LP.
To the point: Live Landmark herself have had CFS/ME, but experienced recovery following a LP course in England. She educated herself to become an LP coach, and have among other things held courses for patients with CFS/ME. The stories of patient recoveries from Recovery Norway, that also include severely ill patients, that have been bedbound and in need of care for years, that become better after a tree day course make an impression. I myself have seen as a GP that a patient went to England to take an LP course (many years ago now - it was not my idea I have never experienced that a patient have had such a large effect from psychotherapy and similar - he came back with a whole new attitude to his symptoms and other challenges in his life that had stolen his energy.) Live Landmark have worked for years to get an RCT on LP, and is a phd candidate at NTNU. I don't know the details of the study (haven't read the protocol in depth etc.), but I am convinced it is important to go through with such a study. Live Landmark has with her a competent set of supervisors, research funding from Forskningsrådet's public phd funds (I think). The study will be done in collaboration with some muncipalities. The study was approved by the regional ethics committee, but was stopped by the national ethics committee after complaints from the ME Association and maybe others.
I was pretty upset about this decision by the national ethics committee. It is of course no doubt that Live Landmark has conflicts of interest, but the economical conflicts of interest must be minimal compared to the pharmaceutical industry. It's thought-provoking that this is talked about as an issue when it comes to public and industry innovation research projects that Forskningsrådet funds. The professional/non-economical conflicts of interests she has is should also be no different than what other researchers have when they research an intervention one believes in. It is hopefully not a requirement that researchers should be devoid of interest in interventions they wish to research. I don't understand that conflicts of interest in the LP study can be ground for not providing ethic approval. Here is a "double standards in research ethics" as Iain Chalmers said on clinical research - he pointed out it is a requirement for consent to take part in an rct where the effect of an intervention is not known, whereas there is no requirement for consent to expose patients to treatments without documented effect in clinical practice (parallell dilemma that we in CEIR has experienced during the pandemic - requirement for consent have stopped many relevant studies on spread of disease). About LP - it is ok to earn money on courses/treatments etc. without ethical approvment, but if one wishes to evaluate the effect in a research design, should ethics stop it? If the money made on LP courses for patients with CFS/ME was what was important, it is unlikely one would waste time doing research.
I have the notion that NEM see the LP study as not serious, with a lacking design and colored by Live Landmarks conflict of interest. A usual complaint on many studies on CFS/ME (also in the work with the NICE guidelines) is that research on interventions it is impossible to blind without primary outcomes that are objective, cannot give trustworthy results/the trust must be downgraded. Even if there of course is bias with subjective symptoms/lack of blinding, this is no different than for a number of other interventions where blinding is impossible (except for those that measures outcomes of ev) - ie. studies on more or less all non-pharmacological interventions, including surgery, physiotherapy, psychotherapy etc. There is growin awareness that PROMs are important. For a condition like CFS/ME, where the diagnosis is based on subjective symptoms. (sic) it is special to insist on objective primary outcomes. It is of course important to try and define objective outcomes, but it is hard to find an objective primary outcome that is important to all patients.
The regional ethics committee has now approved the study again, after some changes where done (again I don't know the details). I have understood that the ME activists again have complained about this decision. I don't know if the national ethics committee will get the study for review again, but I have understood that you are a new member of the national ethics committee. That is the reason I contacted you.
I hope the complaint do not leat to the national ethics committee reviewing the case again (I don't know how this goes). But if the national committee should get the case again, I am as you understand strongly invested in that the national ethics committee don't repeat what I think was a completely hopeless decision.
I can say more about ME activism if it becomes necessary and you are interested. The patient group is in need of hope. The ME activists harass patients that have recovered, and they also harass researchers and others that don't support their strategy.
LP is not for everyone, but for motivated patients I am convinced this is a potential very effective intervention. It is important with more research based knowledge of LP.
the ME activists spreads a lot of misleading claims about what LP is, also dubious stories about harms. Stories about relapses that happens long after the course has been done can hardly be given much weight, for a patient group that experiences that everything they do cause harm (PEM is now a cardinal symptom according to the new NICE guidelines - post-exertional-malaise). Positive and dramatic effects that come immediately after the course must on the other hand be believed to be causal effects.
Now I'm heading out to clean a bit in the forest here at ---- We had snow this weekend - now great spring weather again. --- says hi!
Regards, Signe

