Nightsong
Senior Member (Voting Rights)
Abstract
Background: This study examined the regional distribution of glial activation in essential workers with neurological post-acute sequelae of coronavirus disease 2019 (COVID-19) infections (N-PASC).
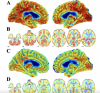
Methods: We injected ≤185 MBq of [18F]-FEPPA as an intravenous bolus and positron-emission tomography over two hours. To measure distribution volume (VT) we acquired an arterial input function in 24 essential workers (14 N-PASC, 10 Never-COVID-19 Controls, of whom eight successfully placed arterial lines). Individuals with low binding affinity were excluded from this study, and VT was adjusted for translocator protein genotype. Analyses that passed the false discovery rate are reported.
Results: Participants at midlife survived mild to moderate COVID-19 without hospitalization but reported onset of post-acute sequelae for, on average, 22 months before undergoing neuroimaging. Hippocampal VT was higher (VT=1.70, 95% C.I. = [1.30-2.21], p=0.001) in participants with persistent brain fog after COVID-19, reflecting an increase of 10.58 ml/cm3 in VT (area under the receiver-operating curve, AUC= 0.95 [0.85-1.00]). At a cutoff of 10.6, sensitivity/specificity/accuracy were 0.88/0.93/0.91.
Conclusion: The results from this study imply that neuroimmune response is a distinct and identifiable characteristic of brain fog after COVID-19. Results suggest that [18F]-FEPPA could be used to support PASC diagnosis.
Link | PDF
Background: This study examined the regional distribution of glial activation in essential workers with neurological post-acute sequelae of coronavirus disease 2019 (COVID-19) infections (N-PASC).
Methods: We injected ≤185 MBq of [18F]-FEPPA as an intravenous bolus and positron-emission tomography over two hours. To measure distribution volume (VT) we acquired an arterial input function in 24 essential workers (14 N-PASC, 10 Never-COVID-19 Controls, of whom eight successfully placed arterial lines). Individuals with low binding affinity were excluded from this study, and VT was adjusted for translocator protein genotype. Analyses that passed the false discovery rate are reported.
Results: Participants at midlife survived mild to moderate COVID-19 without hospitalization but reported onset of post-acute sequelae for, on average, 22 months before undergoing neuroimaging. Hippocampal VT was higher (VT=1.70, 95% C.I. = [1.30-2.21], p=0.001) in participants with persistent brain fog after COVID-19, reflecting an increase of 10.58 ml/cm3 in VT (area under the receiver-operating curve, AUC= 0.95 [0.85-1.00]). At a cutoff of 10.6, sensitivity/specificity/accuracy were 0.88/0.93/0.91.
Conclusion: The results from this study imply that neuroimmune response is a distinct and identifiable characteristic of brain fog after COVID-19. Results suggest that [18F]-FEPPA could be used to support PASC diagnosis.
Link | PDF