Mij
Senior Member (Voting Rights)
Abstract
Life-threatening thrombotic events and neurological symptoms are prevalent in COVID-19 and are persistent in patients with long COVID experiencing post-acute sequelae of SARS-CoV-2 infection1,2,3,4. Despite the clinical evidence1,5,6,7, the underlying mechanisms of coagulopathy in COVID-19 and its consequences in inflammation and neuropathology remain poorly understood and treatment options are insufficient. Fibrinogen, the central structural component of blood clots, is abundantly deposited in the lungs and brains of patients with COVID-19, correlates with disease severity and is a predictive biomarker for post-COVID-19 cognitive deficits1,5,8,9,10.
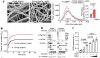
Here we show that fibrin binds to the SARS-CoV-2 spike protein, forming proinflammatory blood clots that drive systemic thromboinflammation and neuropathology in COVID-19. Fibrin, acting through its inflammatory domain, is required for oxidative stress and macrophage activation in the lungs, whereas it suppresses natural killer cells, after SARS-CoV-2 infection.
Fibrin promotes neuroinflammation and neuronal loss after infection, as well as innate immune activation in the brain and lungs independently of active infection. A monoclonal antibody targeting the inflammatory fibrin domain provides protection from microglial activation and neuronal injury, as well as from thromboinflammation in the lung after infection. Thus, fibrin drives inflammation and neuropathology in SARS-CoV-2 infection, and fibrin-targeting immunotherapy may represent a therapeutic intervention for patients with acute COVID-19 and long COVID.
LINK
Life-threatening thrombotic events and neurological symptoms are prevalent in COVID-19 and are persistent in patients with long COVID experiencing post-acute sequelae of SARS-CoV-2 infection1,2,3,4. Despite the clinical evidence1,5,6,7, the underlying mechanisms of coagulopathy in COVID-19 and its consequences in inflammation and neuropathology remain poorly understood and treatment options are insufficient. Fibrinogen, the central structural component of blood clots, is abundantly deposited in the lungs and brains of patients with COVID-19, correlates with disease severity and is a predictive biomarker for post-COVID-19 cognitive deficits1,5,8,9,10.
Here we show that fibrin binds to the SARS-CoV-2 spike protein, forming proinflammatory blood clots that drive systemic thromboinflammation and neuropathology in COVID-19. Fibrin, acting through its inflammatory domain, is required for oxidative stress and macrophage activation in the lungs, whereas it suppresses natural killer cells, after SARS-CoV-2 infection.
Fibrin promotes neuroinflammation and neuronal loss after infection, as well as innate immune activation in the brain and lungs independently of active infection. A monoclonal antibody targeting the inflammatory fibrin domain provides protection from microglial activation and neuronal injury, as well as from thromboinflammation in the lung after infection. Thus, fibrin drives inflammation and neuropathology in SARS-CoV-2 infection, and fibrin-targeting immunotherapy may represent a therapeutic intervention for patients with acute COVID-19 and long COVID.
LINK