Chandelier
Senior Member (Voting Rights)
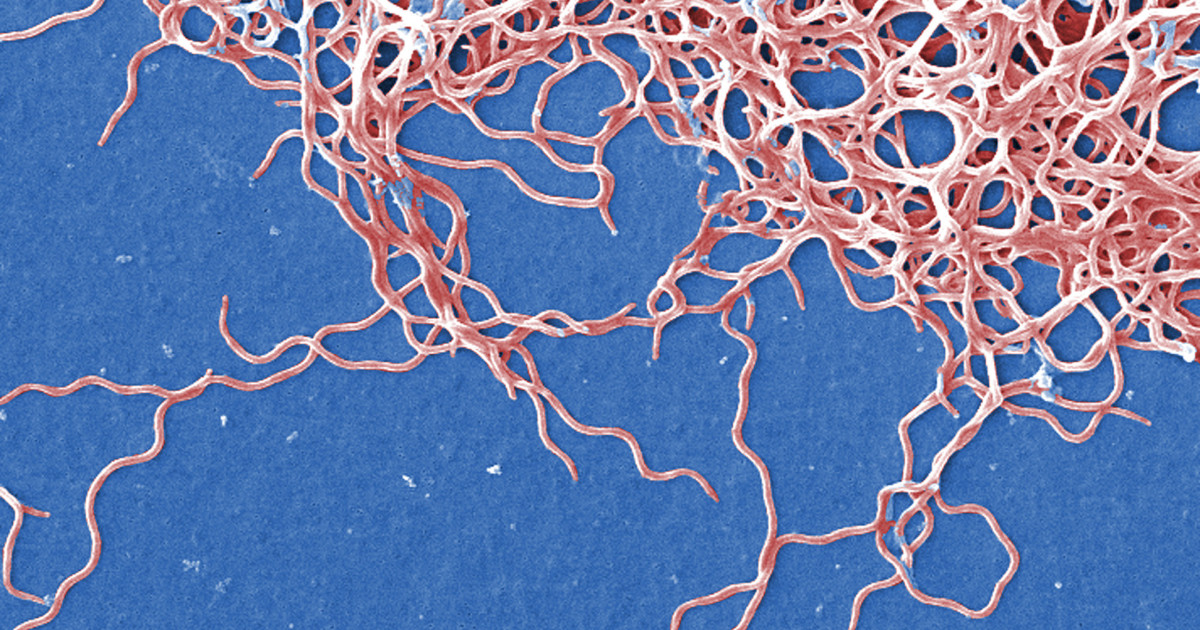
EPR spectroscopy reveals antioxidant manganese defenses in the Lyme disease pathogen Borrelia burgdorferi
We investigated these antioxidant systems in Borrelia burgdorferi, the Mn-accumulating, Fe-independent Lyme disease pathogen.
Using electron paramagnetic resonance (EPR) and electron-nuclear double resonance (ENDOR) spectroscopies, we tracked Mn²+ partitioning between enzyme-bound (L-Mn) and metabolite-bound (H-Mn) pools in spirochetes at exponential and stationary phases.
Results show that MnSOD neutralizes extracellular O2•− generated by γ-irradiation (a model for host immune attack); H-Mn neutralizes cytoplasmic O2•− and is a reservoir of labile Mn²+for metalating Mn-dependent enzymes. MnCl2supplementation in log phase B. burgdorferirestored radioresistance in ΔMnSOD mutants via H-Mn hyperaccumulation but induced toxicity in older, stationary phase cells as metabolites became depleted.
These findings support an expanded oxidative-stress model in which H-Mn complements MnSOD and positions Mn homeostasis as a therapeutic target. Our approach highlights the utility of EPR and ENDOR in studying Mn-dependent pathogens.
We measured the spirochete’s survivability to acute γ-irradiation, which simulates the respiratory burst of O2•− deployed as a critical weapon in the host’s innate immune response.
While Mn-superoxide dismutase (MnSOD) has classically been viewed as the main defense against oxidative damage in B. burgdorferi, our study demonstrates that antioxidant Mn2+ complexes with the metabolite components of H-Mn play a crucial antioxidant role, particularly when MnSOD is deficient.
However, B. burgdorferi’s inability to safely store excess Mn in metabolite-depleted cells highlights novel metabolic vulnerabilities that could be exploited for managing Lyme disease.
Web | DOI | PDF | mBio
Londoño, Andrés F.; Sharma, Ajay; Kathiresan, Venkatesan; Sealy, Jared; Volpe, Robert P.; Gostinčar, Cene; Pal, Utpal; Dumler, J. Stephen; Hoffman, Brian M.; Daly, Michael J.
ABSTRACT
Oxidative stress defense in aerobic bacteria relies on Mn-superoxide dismutase (MnSOD) and antioxidant Mn-metabolite complexes (H-Mn) to quench superoxide radicals (O2•−).We investigated these antioxidant systems in Borrelia burgdorferi, the Mn-accumulating, Fe-independent Lyme disease pathogen.
Using electron paramagnetic resonance (EPR) and electron-nuclear double resonance (ENDOR) spectroscopies, we tracked Mn²+ partitioning between enzyme-bound (L-Mn) and metabolite-bound (H-Mn) pools in spirochetes at exponential and stationary phases.
Results show that MnSOD neutralizes extracellular O2•− generated by γ-irradiation (a model for host immune attack); H-Mn neutralizes cytoplasmic O2•− and is a reservoir of labile Mn²+for metalating Mn-dependent enzymes. MnCl2supplementation in log phase B. burgdorferirestored radioresistance in ΔMnSOD mutants via H-Mn hyperaccumulation but induced toxicity in older, stationary phase cells as metabolites became depleted.
These findings support an expanded oxidative-stress model in which H-Mn complements MnSOD and positions Mn homeostasis as a therapeutic target. Our approach highlights the utility of EPR and ENDOR in studying Mn-dependent pathogens.
IMPORTANCE
We employed electron paramagnetic resonance and electron-nuclear double resonance spectroscopies of Mn²+ in intact Borrelia burgdorferi supplemented with MnCl2 to track changes in the amounts of enzyme-bound Mn and substitutionally labile, antioxidant Mn-metabolite complexes.We measured the spirochete’s survivability to acute γ-irradiation, which simulates the respiratory burst of O2•− deployed as a critical weapon in the host’s innate immune response.
While Mn-superoxide dismutase (MnSOD) has classically been viewed as the main defense against oxidative damage in B. burgdorferi, our study demonstrates that antioxidant Mn2+ complexes with the metabolite components of H-Mn play a crucial antioxidant role, particularly when MnSOD is deficient.
However, B. burgdorferi’s inability to safely store excess Mn in metabolite-depleted cells highlights novel metabolic vulnerabilities that could be exploited for managing Lyme disease.
Web | DOI | PDF | mBio