Andy
Senior Member (Voting rights)
Abstract
Objective To describe the development and validation of a novel patient reported outcome measure for symptom burden from long covid, the symptom burden questionnaire for long covid (SBQ-LC).
Design Multiphase, prospective mixed methods study.
Setting Remote data collection and social media channels in the United Kingdom, 14 April to 1 August 2021.
Participants 13 adults (aged ≥18 years) with self-reported long covid and 10 clinicians evaluated content validity. 274 adults with long covid field tested the draft questionnaire.
Main outcome measures Published systematic reviews informed development of SBQ-LC’s conceptual framework and initial item pool. Thematic analysis of transcripts from cognitive debriefing interviews and online clinician surveys established content validity. Consensus discussions with the patient and public involvement group of the Therapies for Long COVID in non-hospitalised individuals: From symptoms, patient reported outcomes and immunology to targeted therapies (TLC Study) confirmed face validity. Rasch analysis of field test data guided item and scale refinement and provided initial evidence of the SBQ-LC’s measurement properties.
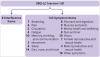
Results SBQ-LC (version 1.0) is a modular instrument measuring patient reported outcomes and is composed of 17 independent scales with promising psychometric properties. Respondents rate their symptom burden during the past seven days using a dichotomous response or 4 point rating scale. Each scale provides coverage of a different symptom domain and returns a summed raw score that can be transformed to a linear (0-100) score. Higher scores represent higher symptom burden. After rating scale refinement and item reduction, all scales satisfied the Rasch model requirements for unidimensionality (principal component analysis of residuals: first residual contrast values <2.00 eigenvalue units) and item fit (outfit mean square values within 0.5 -1.5 logits). Rating scale categories were ordered with acceptable category fit statistics (outfit mean square values <2.0 logits). 14 item pairs had evidence of local dependency (residual correlation values >0.4). Across the 17 scales, person reliability ranged from 0.34 to 0.87, person separation ranged from 0.71 to 2.56, item separation ranged from 1.34 to 13.86, and internal consistency reliability (Cronbach’s alpha) ranged from 0.56 to 0.91.
Conclusions SBQ-LC (version 1.0) is a comprehensive patient reported outcome instrument developed using modern psychometric methods. It measures symptoms of long covid important to people with lived experience of the condition and may be used to evaluate the impact of interventions and inform best practice in clinical management.
Open access, https://www.bmj.com/content/377/bmj-2022-070230
Objective To describe the development and validation of a novel patient reported outcome measure for symptom burden from long covid, the symptom burden questionnaire for long covid (SBQ-LC).
Design Multiphase, prospective mixed methods study.
Setting Remote data collection and social media channels in the United Kingdom, 14 April to 1 August 2021.
Participants 13 adults (aged ≥18 years) with self-reported long covid and 10 clinicians evaluated content validity. 274 adults with long covid field tested the draft questionnaire.
Main outcome measures Published systematic reviews informed development of SBQ-LC’s conceptual framework and initial item pool. Thematic analysis of transcripts from cognitive debriefing interviews and online clinician surveys established content validity. Consensus discussions with the patient and public involvement group of the Therapies for Long COVID in non-hospitalised individuals: From symptoms, patient reported outcomes and immunology to targeted therapies (TLC Study) confirmed face validity. Rasch analysis of field test data guided item and scale refinement and provided initial evidence of the SBQ-LC’s measurement properties.
Results SBQ-LC (version 1.0) is a modular instrument measuring patient reported outcomes and is composed of 17 independent scales with promising psychometric properties. Respondents rate their symptom burden during the past seven days using a dichotomous response or 4 point rating scale. Each scale provides coverage of a different symptom domain and returns a summed raw score that can be transformed to a linear (0-100) score. Higher scores represent higher symptom burden. After rating scale refinement and item reduction, all scales satisfied the Rasch model requirements for unidimensionality (principal component analysis of residuals: first residual contrast values <2.00 eigenvalue units) and item fit (outfit mean square values within 0.5 -1.5 logits). Rating scale categories were ordered with acceptable category fit statistics (outfit mean square values <2.0 logits). 14 item pairs had evidence of local dependency (residual correlation values >0.4). Across the 17 scales, person reliability ranged from 0.34 to 0.87, person separation ranged from 0.71 to 2.56, item separation ranged from 1.34 to 13.86, and internal consistency reliability (Cronbach’s alpha) ranged from 0.56 to 0.91.
Conclusions SBQ-LC (version 1.0) is a comprehensive patient reported outcome instrument developed using modern psychometric methods. It measures symptoms of long covid important to people with lived experience of the condition and may be used to evaluate the impact of interventions and inform best practice in clinical management.
Open access, https://www.bmj.com/content/377/bmj-2022-070230