Chandelier
Senior Member (Voting Rights)
Beyond macrophages: FIPV tropism includes T and B lymphocytes
While the prevailing model holds that FIPV selectively infects monocytes/macrophages, the full range of susceptible cell types and the mechanisms of immune cell invasion remain poorly defined.
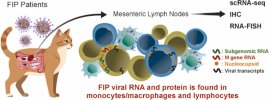
Here, we applied single-cell RNA sequencing, multiplex immunofluorescence, and in situhybridization to mesenteric lymph node aspirates and formalin fixed and paraffin embedded lymph node tissues from cats with naturally occurring effusive FIP.
We identified FIPV RNA and nucleocapsid protein in T and B lymphocytes and myeloid cells, and subgenomic viral RNA in T cells, demonstrating cell entry and viral genomic replication across multiple immune compartments.
Rare FIPV RNA–positive lymphocytes persisted after antiviral treatment cessation and resolution of clinical signs.
These findings revise current models of FIPV pathogenesis and reveal new insights into coronavirus-driven immune dysregulation, viral persistence, and relapse.
Our study highlights the utility of FIP as a naturally occurring animal model for exploring adaptive immune cell infection in coronavirus diseases, providing a translational platform for understanding virus–host interactions that drive chronic or relapsing immunopathology.
Web | DOI | Veterinary Microbiology
Balakumar, Aadhavan; Wanakumjorn, Patrawin; Kimura, Kazuto; McLarty, Ehren; Farrell, Katherine; Brostoff, Terza; Pires, Jully; Cohen-Davidyan, Tamar; Cassano, Jennifer M.; Murphy, Brian; Reagan, Krystle; Kol, Amir
Highlights
- FIPV infects monocytes/macrophages and also enters T and B lymphocytes in cats with effusive FIP.
- Single-cell RNA sequencing and confocal imaging reveal FIPV RNA and nucleocapsid protein in T and B lymphocytes.
- Subgenomic FIPV RNA is detected in CD3⁺ T, indicating active viral RNA replication.
- FIPV RNA–positive lymphocytes uniquely activate antiviral and innate immune pathways, supporting true cellular infection.
- Low-level FIPV RNA persists in lymphocytes after antiviral therapy despite clinical resolution.
Abstract
If untreated, feline infectious peritonitis (FIP) is a fatal disease that is caused by feline infectious peritonitis virus (FIPV), a virulent biotype of feline coronavirus (FCoV) that disseminates broadly and triggers severe systemic inflammation.While the prevailing model holds that FIPV selectively infects monocytes/macrophages, the full range of susceptible cell types and the mechanisms of immune cell invasion remain poorly defined.
Here, we applied single-cell RNA sequencing, multiplex immunofluorescence, and in situhybridization to mesenteric lymph node aspirates and formalin fixed and paraffin embedded lymph node tissues from cats with naturally occurring effusive FIP.
We identified FIPV RNA and nucleocapsid protein in T and B lymphocytes and myeloid cells, and subgenomic viral RNA in T cells, demonstrating cell entry and viral genomic replication across multiple immune compartments.
Rare FIPV RNA–positive lymphocytes persisted after antiviral treatment cessation and resolution of clinical signs.
These findings revise current models of FIPV pathogenesis and reveal new insights into coronavirus-driven immune dysregulation, viral persistence, and relapse.
Our study highlights the utility of FIP as a naturally occurring animal model for exploring adaptive immune cell infection in coronavirus diseases, providing a translational platform for understanding virus–host interactions that drive chronic or relapsing immunopathology.
Graphical Abstract

Web | DOI | Veterinary Microbiology

