Chandelier
Senior Member (Voting Rights)
Thorsten Lehr, PhD; Peter Meiser, PhD; Dominik Selzer, PhD
Key Points
Question Is regular application of azelastine nasal spray associated with reduced risk of SARS-CoV-2 infections?
Findings In this randomized placebo-controlled clinical trial that included 450 participants, the incidence of laboratory-confirmed SARS-CoV-2 infections was significantly lower with application of azelastine nasal spray compared with placebo treatment.
Meaning The use of azelastine nasal spray may help to reduce the risk of SARS-CoV-2 infections.
Abstract
Importance Limited pharmaceutical options exist for preexposure prophylaxis of COVID-19 beyond vaccination. Azelastine, an antihistamine nasal spray used for decades to treat allergic rhinitis, has in vitro antiviral activity against respiratory viruses, including SARS-CoV-2.
Objective To determine the efficacy and safety of azelastine nasal spray for prevention of SARS-CoV-2 infections in healthy adults.
Design, Setting, and Participants A phase 2, double-blind, placebo-controlled, single-center trial was conducted from March 2023 to July 2024. Healthy adults from the general population were enrolled at the Saarland University Hospital in Germany.
Interventions Participants were randomly assigned 1:1 to receive azelastine, 0.1%, nasal spray or placebo 3 times daily for 56 days. SARS-CoV-2 rapid antigen testing (RAT) was conducted twice weekly, with positive results confirmed by polymerase chain reaction (PCR). Symptomatic participants with negative RAT results underwent multiplex PCR testing for respiratory viruses.
Main Outcome The primary end point was the number of PCR-confirmed SARS-CoV-2 infections during the study.
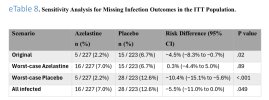
Results A total of 450 participants were randomized, with 227 assigned to azelastine and 223 to placebo; 299 (66.4%) were female, 151 (33.6%) male, with a mean (SD) age of 33.0 (13.3) years. Most were White (417 [92.7%]), with 4 (0.9%) African, 22 (4.9%) Asian, and 7 (1.6%) of other ethnicity. In the intention-to-treat (ITT) population, the incidence of PCR-confirmed SARS-CoV-2 infection was significantly lower in the azelastine group (n = 5 [2.2%]) compared with the placebo group (n = 15 [6.7%]) (OR, 0.31; 95% CI, 0.11-0.87). As secondary end points, azelastine demonstrated an increase in mean (SD) time to SARS-CoV-2 infection among infected participants (31.2 [9.3] vs 19.5 [14.8] days), a reduction of the overall number of PCR-confirmed symptomatic infections (21 of 227 participants vs 49 of 223 participants), and a lower incidence of PCR-confirmed rhinovirus infections (1.8% vs 6.3%). Adverse events were comparable between the groups.
Conclusions and Relevance In this single-center trial, azelastine nasal spray was associated with reduced risk of SARS-CoV-2 respiratory infections. These findings support the potential of azelastine as a safe prophylactic approach warranting confirmation in larger, multicentric trials.
Dr Lehr reported grants from Ursapharm as a study sponsor during the conduct of the study;
personal fees from Saarmetrics GmbH as a founder and shareholder outside the submitted work.
Dr Meiser reported personal fees from URSAPHARM Arzneimittel GmbH for employment outside the submitted work;
and Dr Meiser is employed at URSAPHARM Arzneimittel GmbH, the sponsor of the CONTAIN trial.
Dr Selzer reported grants from URSAPHARM Arzneimittel GmbH as a study sponsor during the conduct of the study;
grants from the Scientific Consilience GmbH for constultant work for the company outside the submitted work.
Dr Holzer reported personal fees from URSAPHARM Arzneimittel GmbH as CEO of the company outside the submitted work;
and Frank Holzer is the CEO of URSAPHARM Arzneimittel GmbH, the sponsor of the CONTAIN trial.
Dr Mösges reported personal fees from Ursapharm GmbH and grants from Ursapharm GmbH during the conduct of the study; personal fees from ALK, grants from ASITbiotech, personal fees from Allergopharma, personal fees from Bencard, grants from Leti, grants from Lofarma, nonfinancial support from Roxall, personal fees from Stallergenes, grants from Bencard, personal fees from Lofarma, nonfinancial support from Lofarma, grants from Stallergenes, personal fees from Optima, Friulchem, Hexai, Servier, and Klosterfrau, nonfinancial support from Atmos, personal fees from Bayer, nonfinancial support from Bionorica, personal fees from FAES, GSK, MSD, Johnson & Johnson, Meda, and Novartis, nonfinancial support from Novartis, nonfinancial support from Otonomy, personal fees from Stada and UCB, nonfinancial support from Ferrero, grants from Hulka, personal fees from Nuvo, Menarini, Mundipharma, and Pohl-Boskamp, grants from Inmunotek, personal fees from Cassella-med GmbH&Co KG, Laboratoire de la Mer, and Sidroga, grants from HAL BV, personal fees from HAL BV, Lek, PRO-AdWISE, Angelini Pharma, and JGL, nonfinancial support from JGL, grants from bitop, personal fees from bitop and Sanofi, grants from Probelte Pharma, personal fees from Probelte Pharma, Diater, and Worg Pharma, grants from Allergy Therapeutics, and personal fees from Allergy Therapeutics outside the submitted work.
Dr Smola reported grants from URSAPHARM Arzneimittel GmbH as institutional funding to perform laboratory analysis for the present study during the conduct of the study.
Dr Bals reported grants from Ursapharm Pharmaceuticals during the conduct of the study;
grants from Deutsche Forschungsgemeinschaft, German Ministry for Research and Education, Schwiete-Foundation, and the State of Saarland, personal fees from CSL Behring, Grifols, AstraZeneca, GSK, and Regeneron outside the submitted work. No other disclosures were reported.
personal fees from Saarmetrics GmbH as a founder and shareholder outside the submitted work.
Dr Meiser reported personal fees from URSAPHARM Arzneimittel GmbH for employment outside the submitted work;
and Dr Meiser is employed at URSAPHARM Arzneimittel GmbH, the sponsor of the CONTAIN trial.
Dr Selzer reported grants from URSAPHARM Arzneimittel GmbH as a study sponsor during the conduct of the study;
grants from the Scientific Consilience GmbH for constultant work for the company outside the submitted work.
Dr Holzer reported personal fees from URSAPHARM Arzneimittel GmbH as CEO of the company outside the submitted work;
and Frank Holzer is the CEO of URSAPHARM Arzneimittel GmbH, the sponsor of the CONTAIN trial.
Dr Mösges reported personal fees from Ursapharm GmbH and grants from Ursapharm GmbH during the conduct of the study; personal fees from ALK, grants from ASITbiotech, personal fees from Allergopharma, personal fees from Bencard, grants from Leti, grants from Lofarma, nonfinancial support from Roxall, personal fees from Stallergenes, grants from Bencard, personal fees from Lofarma, nonfinancial support from Lofarma, grants from Stallergenes, personal fees from Optima, Friulchem, Hexai, Servier, and Klosterfrau, nonfinancial support from Atmos, personal fees from Bayer, nonfinancial support from Bionorica, personal fees from FAES, GSK, MSD, Johnson & Johnson, Meda, and Novartis, nonfinancial support from Novartis, nonfinancial support from Otonomy, personal fees from Stada and UCB, nonfinancial support from Ferrero, grants from Hulka, personal fees from Nuvo, Menarini, Mundipharma, and Pohl-Boskamp, grants from Inmunotek, personal fees from Cassella-med GmbH&Co KG, Laboratoire de la Mer, and Sidroga, grants from HAL BV, personal fees from HAL BV, Lek, PRO-AdWISE, Angelini Pharma, and JGL, nonfinancial support from JGL, grants from bitop, personal fees from bitop and Sanofi, grants from Probelte Pharma, personal fees from Probelte Pharma, Diater, and Worg Pharma, grants from Allergy Therapeutics, and personal fees from Allergy Therapeutics outside the submitted work.
Dr Smola reported grants from URSAPHARM Arzneimittel GmbH as institutional funding to perform laboratory analysis for the present study during the conduct of the study.
Dr Bals reported grants from Ursapharm Pharmaceuticals during the conduct of the study;
grants from Deutsche Forschungsgemeinschaft, German Ministry for Research and Education, Schwiete-Foundation, and the State of Saarland, personal fees from CSL Behring, Grifols, AstraZeneca, GSK, and Regeneron outside the submitted work. No other disclosures were reported.