LarsSG
Senior Member (Voting Rights)
This is the preprint. See post #4 for the published paper
Augmentation of Anaerobic Pentose Phosphate Pathway Dysregulates Tetrahydrobiopterin Metabolism in Myalgic Encephalomyelitis/ Chronic Fatigue Syndrome (ME/CFS) Patients with Orthostatic Intolerance: A Pilot Study
Sarojini Bulbule, Carl Gunnar Gottschalk, Molly E Drosen, Daniel Peterson, Leggy A Arnold, Avik Roy
Tetrahydrobiopterin (BH4), an essential cofactor of amino acid metabolism, was found to be strongly elevated in ME/CFS patients with Orthostatic intolerance (ME + OI). However, the molecular mechanism of BH4 upregulation is poorly understood in ME + OI patients.
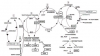
Here, we report that the activation of the non-oxidative pentose phosphate pathway (PPP) plays a critical role in the biosynthesis of BH4 in ME + OI patients. Microarray-based gene screening followed by real-time PCR-based validation, ELISA assay, and finally enzyme kinetic studies of glucose-6-phosphate dehydrogenase (G6PDH), transaldolase (TALDO1), and transketolase (TK) enzymes revealed that the augmentation of anaerobic PPP is critical in the pathogenesis of ME + OI.
Along with the upregulated anaerobic PPP enzymes, we observed that biopterin metabolites such as BH4 and dihydrobiopterin (BH2) are strongly upregulated suggesting the disruption of biopterin homeostasis in ME + OI patients. To explore the molecular role of anaerobic PPP in biopterin metabolism, we devised a novel cell culture strategy to induce non-oxidative PPP by treating human microglial cells with ribose-5-phosphate (R5P) under a hypoxic condition of 85%N2/10%CO2/5%O2 followed by the analysis of BH4 and BH2 upregulation via ELISA, immunoblot and dual immunocytochemical analyses. These results confirmed that the activation of non-oxidative PPP is indeed required for the upregulation of both BH4 and BH2.
Moreover, the siRNA knocking down of the taldo1 gene strongly inhibited the expression of GTP cyclohydrolase 1 (GTPCH1) and subsequent production of BH4 and its metabolic conversion to BH2 in R5P-treated and hypoxia-induced C20 human microglia cells. To test the functional role of ME + OI plasma-derived biopterins, exogenously added plasma samples of ME + OI plasma with high BH4 upregulated inducible nitric oxide synthase (iNOS) and nitric oxide (NO) in human microglial cells indicating that the non-oxidative PPP-induced-biopterins could stimulate inflammatory response in ME + OI patients.
https://www.researchsquare.com/article/rs-3716093/v1
Augmentation of Anaerobic Pentose Phosphate Pathway Dysregulates Tetrahydrobiopterin Metabolism in Myalgic Encephalomyelitis/ Chronic Fatigue Syndrome (ME/CFS) Patients with Orthostatic Intolerance: A Pilot Study
Sarojini Bulbule, Carl Gunnar Gottschalk, Molly E Drosen, Daniel Peterson, Leggy A Arnold, Avik Roy
Tetrahydrobiopterin (BH4), an essential cofactor of amino acid metabolism, was found to be strongly elevated in ME/CFS patients with Orthostatic intolerance (ME + OI). However, the molecular mechanism of BH4 upregulation is poorly understood in ME + OI patients.
Here, we report that the activation of the non-oxidative pentose phosphate pathway (PPP) plays a critical role in the biosynthesis of BH4 in ME + OI patients. Microarray-based gene screening followed by real-time PCR-based validation, ELISA assay, and finally enzyme kinetic studies of glucose-6-phosphate dehydrogenase (G6PDH), transaldolase (TALDO1), and transketolase (TK) enzymes revealed that the augmentation of anaerobic PPP is critical in the pathogenesis of ME + OI.
Along with the upregulated anaerobic PPP enzymes, we observed that biopterin metabolites such as BH4 and dihydrobiopterin (BH2) are strongly upregulated suggesting the disruption of biopterin homeostasis in ME + OI patients. To explore the molecular role of anaerobic PPP in biopterin metabolism, we devised a novel cell culture strategy to induce non-oxidative PPP by treating human microglial cells with ribose-5-phosphate (R5P) under a hypoxic condition of 85%N2/10%CO2/5%O2 followed by the analysis of BH4 and BH2 upregulation via ELISA, immunoblot and dual immunocytochemical analyses. These results confirmed that the activation of non-oxidative PPP is indeed required for the upregulation of both BH4 and BH2.
Moreover, the siRNA knocking down of the taldo1 gene strongly inhibited the expression of GTP cyclohydrolase 1 (GTPCH1) and subsequent production of BH4 and its metabolic conversion to BH2 in R5P-treated and hypoxia-induced C20 human microglia cells. To test the functional role of ME + OI plasma-derived biopterins, exogenously added plasma samples of ME + OI plasma with high BH4 upregulated inducible nitric oxide synthase (iNOS) and nitric oxide (NO) in human microglial cells indicating that the non-oxidative PPP-induced-biopterins could stimulate inflammatory response in ME + OI patients.
https://www.researchsquare.com/article/rs-3716093/v1
Last edited by a moderator: