Abstract
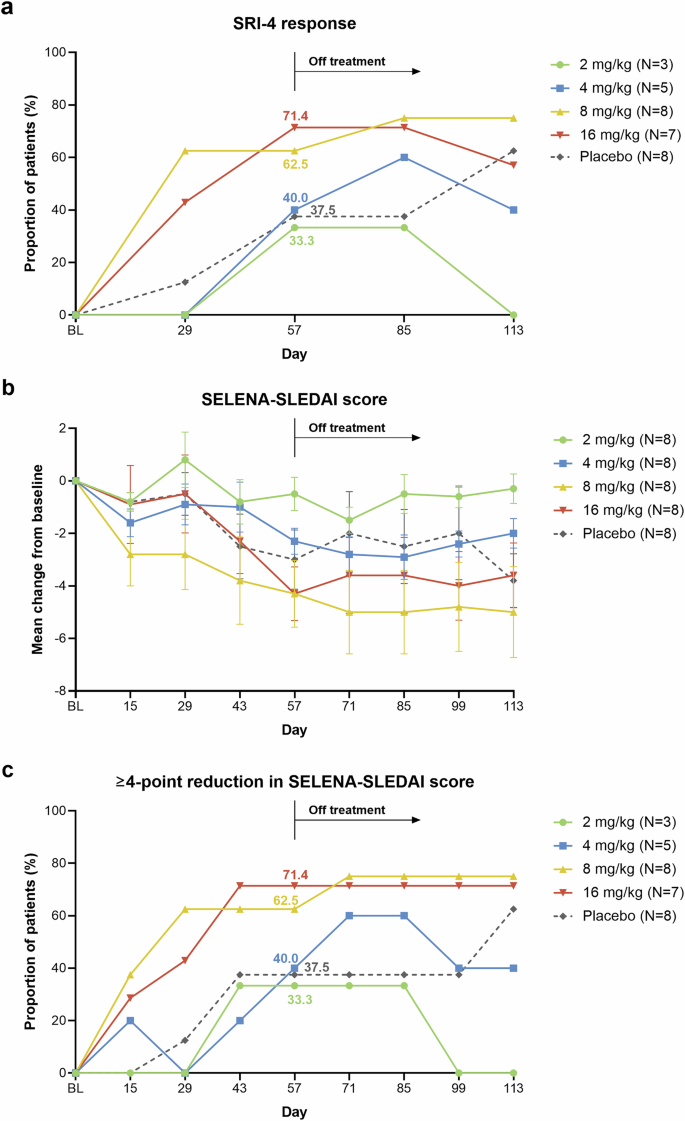
CD38 is highly expressed on various immune cells, including long-lived plasma cells, making it a potential therapeutic target in autoimmune diseases. This phase Ib/IIa study aimed to explore the safety, pharmacokinetics, pharmacodynamics, and preliminary efficacy of CM313, an anti-CD38 antibody, in patients with systemic lupus erythematosus (SLE). Eligible patients were sequentially enrolled in four ascending dose groups (2, 4, 8, and 16 mg/kg) and randomized 4:1 to receive CM313 or placebo intravenously at days 1, 29, 36, 43, and 50. The primary endpoint was safety, and efficacy was exploratorily investigated. Between October 14, 2022, and March 7, 2024, 40 patients were enrolled, including 8 patients in each CM313 dose group and the pooled placebo group. Adverse events occurred in 90.6% and 62.5% of participants receiving CM313 and placebo, all of which were mild or moderate. Upper respiratory tract infection (87.5%/62.5% vs. 12.5%), urinary tract infection (12.5%/25.0% vs. 0), and herpes zoster (25.0%/0 vs. 0) were more frequent in CM313 8 and 16 mg/kg groups than the placebo group. The CM313 groups had greater reductions in anti-ds-DNA antibodies, immunoglobulin G (IgG), IgA, IgM, IgE, and greater increases in complement C3 and C4 compared with placebo. Systemic Lupus Erythematosus Responder Index-4 response rates were 33.3%, 40.0%, 62.5%, 71.4%, and 37.5% in CM313 2, 4, 8, 16 mg/kg, and placebo groups at day 57, respectively. CM313 showed a manageable safety profile in SLE patients at 2–16 mg/kg and encouraging clinical efficacy at 8 and 16 mg/kg. The results support further investigation of CM313 for treating SLE patients (ClinicalTrials.gov ID: NCT05465707).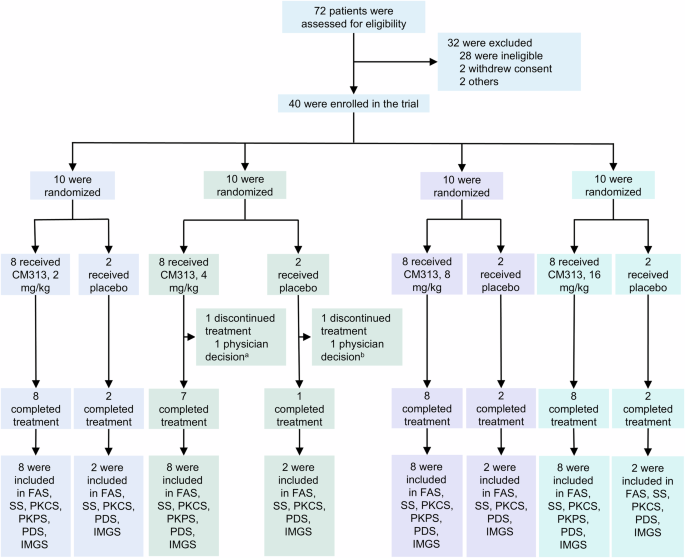
Anti-CD38 monoclonal antibody CM313 for systemic lupus erythematosus: a randomized, double-blind, placebo-controlled phase Ib/IIa trial - Signal Transduction and Targeted Therapy
CD38 is highly expressed on various immune cells, including long-lived plasma cells, making it a potential therapeutic target in autoimmune diseases. This phase Ib/IIa study aimed to explore the safety, pharmacokinetics, pharmacodynamics, and preliminary efficacy of CM313, an anti-CD38 antibody...