Aβ low threshold mechanoreceptors contribute to sensory abnormalities in fibromyalgia
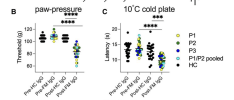
Fibromyalgia syndrome (FM) is characterized by widespread pain and fatigue. People living with FM also experience tactile allodynia, cold-evoked pain, paraesthesia and dysaesthesia. There is evidence of small fibre neuropathy and hyperexcitability of nociceptors in FM; however, the presence of other sensory abnormalities suggests involvement of large diameter sensory fibres. The passive transfer of FM IgG to mice causes cold and mechanical hyperalgesia associated with changes in A- and C-nociceptor function. However, whether FM IgG also confers sensitivity to light touch and whether large diameter sensory fibres contribute to symptoms evoked by cold is unknown.
Here we demonstrate that the presence of sensory abnormalities such as tingling, correlate with the impact of FM, and that people with FM describe the sensation of cutaneous cooling with neuropathic descriptors such as tingling/pins and needles. We find a causal link between circulating FM IgG and the sensitization of large diameter, Aβ low threshold mechanoreceptors (Aβ-LTMRs) to mechanical and cold stimuli in mice ex vivo and in vivo. In keeping with our experimental observations, a larger proportion of Aβ-LTMRs respond to cold stimulation in people with FM, but in contrast to our results ex vivo, the same fibres display reduced responses to mechanical stimuli.
These results expand the pathophysiological role of IgG in FM and will inform future studies of sensory symptoms and pain in people with FM.
Web | DOI | PDF | Brain | Open Access
Israel, Mathilde R; Berwick, Richard; Vastani, Nisha; Zheng, Qin; Moore, Warren; Maurer, Margot; Gentry, Clive; Marshall, Anne; Sun, Haoyue; Neiland, Harvey; Dunham, James P; Bouchatta, Otmane; Plant, Katy; Nagi, Saad S; Olausson, Håkan; Alam, Uazman; Dong, Xinzhong; Bevan, Stuart; Marshall, Andrew; Goebel, Andreas; Andersson, David A
Fibromyalgia syndrome (FM) is characterized by widespread pain and fatigue. People living with FM also experience tactile allodynia, cold-evoked pain, paraesthesia and dysaesthesia. There is evidence of small fibre neuropathy and hyperexcitability of nociceptors in FM; however, the presence of other sensory abnormalities suggests involvement of large diameter sensory fibres. The passive transfer of FM IgG to mice causes cold and mechanical hyperalgesia associated with changes in A- and C-nociceptor function. However, whether FM IgG also confers sensitivity to light touch and whether large diameter sensory fibres contribute to symptoms evoked by cold is unknown.
Here we demonstrate that the presence of sensory abnormalities such as tingling, correlate with the impact of FM, and that people with FM describe the sensation of cutaneous cooling with neuropathic descriptors such as tingling/pins and needles. We find a causal link between circulating FM IgG and the sensitization of large diameter, Aβ low threshold mechanoreceptors (Aβ-LTMRs) to mechanical and cold stimuli in mice ex vivo and in vivo. In keeping with our experimental observations, a larger proportion of Aβ-LTMRs respond to cold stimulation in people with FM, but in contrast to our results ex vivo, the same fibres display reduced responses to mechanical stimuli.
These results expand the pathophysiological role of IgG in FM and will inform future studies of sensory symptoms and pain in people with FM.
Web | DOI | PDF | Brain | Open Access