You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
2023 Health Rising: The Long COVID Clinical Trials: Big Drugs, Big Studies…and More
- Thread starter Jaybee00
- Start date
-
- Tags
- blog cort johnson
First up in Cort's list of trials is American Cryosystem Corporation's stem cell trial. It's described with no concerns expressed at all.
I've made a thread about stem cells treatments and American Cryosystem Corporation here:
Stem cells as treatments
I've made a thread about stem cells treatments and American Cryosystem Corporation here:
Stem cells as treatments
Peter T
Senior Member (Voting Rights)
I think Health Rising is a potentially useful source of information, Cort certainly works hard at gathering information, however that is as long as one does not take the Blog’s reporting at face value and looks critically at the source material. Given it is always well referenced I don’t see its enthusiasm as a problem for the sceptical reader. However the worry is that uncritical readers may get carried away.
Second on Cort's list is this trial:
Impact of Monoclonal Antibody Treatment on Post-Acute COVID-19 Syndrome (MAbPACs)
specifically casirivimab-imdevimab antibody infusion, anti-SARS-CoV-2 monoclonal antibodies that were given during acute Covid-19 treatment.
Summary: This treatment gives people the same sort of antibodies against SARS-CoV-2 that their bodies would produce if vaccinated. The trial aims to see if this treatment administered during the acute disease might reduce post-infection symptoms i.e. there it prevents any of the conditions lumped under the Long Covid name. In the trial design, there is a lack of differentiation between symptoms caused by various post-Covid conditions. Otherwise, it looks like an ok trial.
We know people still get post-Covid symptoms when they have SARS-CoV-2 antibodies from vaccinations, so, while this treatment might help, it's unlikely to have a massive impact on incidence.
This study is not trying to treat established Long Covid.
*****
This retrospective study is being undertaken by Intermountain Healthcare in association with Regeneron Pharmaceuticals. It is wanting to see what impact giving a treatment during the acute illness that aims to reduce the risk of severe Covid-19 has on reporting of symptoms at the 4 to 5 month mark. Controls are to be matched on gender, age and Covid-19 risk.
According to Wikipedia, the casirivimab/imdevimab combo was only to be given to people who were at high risk for progression to severe Covid-19. It does sound as though these antibodies are useful in reducing the severity of the acute disease. As I understand it, these antibodies to the spike protein are of the sort that people would produce themselves when vaccinated, or during a Covid-19 infection.
The Clinical Trials entry says the study is not yet recruiting, although the start date was Aug 2021 and the expected end date was April 2022. So, I'm not sure how active the trial is.
The researchers don't seem to have a very good idea about the mix of health conditions that are bundled up in 'PACS' or Long Covid. There's talk about higher rates of Long Covid among people who are hospitalised, obese and have co-morbidities. It's a bit like doing a study of ME/CFS using a Fukuda diagnostic criteria, except that there is an even greater potential for heterogeneous cohorts. A mixed cohort potentially is ok for answering the specific question - 'does antibody treatment during the acute phase reduce post-Covid symptoms?', so long as researchers go into the study with their eyes open and are able to report on subgroups. I don't see any evidence of an awareness of the likely different symptom groups (e.g. ME/CFS; lung or heart damage), or any plans to group people with different types of post-Covid symptoms in the analysis. The primary outcome is a single 'Post-acute Covid-19 symptom score' - a number out of 60.
The secondary outcomes are have a lot of focus on mental health and on heath care costs e.g. number medically attended visits and health care costs between day 120 and day 150 after date of positive test; measures of depression, anxiety and post-traumatic stress disorder. There are some measures of quality of life and function.
I think this is a reasonable trial as far as it goes. I like that Intermountain Healthcare is making use of its database to evaluate the impact of an acute treatment on longer term outcomes. I don't see any evidence of them working with people who understand what Long Covid actually consists of. And I feel slightly concerned that the researchers will find that obese people with co-morbidities including depression and anxiety are more likely to report post-Covid symptoms and that it will be assumed that that finding applies also to people with post-Covid-19 ME/CFS.
If it's really true that vaccination does reduce the risk of post-Covid-19 ME/CFS (as opposed to just post-Covid symptoms in general), then I suppose that it's conceivable that this treatment (which supplies anti-spoke antibodies rather than inducing the person's body to make them itself) could also reduce the risk.
I'm not sure Cort fully understands what different monoclonal antibodies do. I don't know myself, but surely the action of Rituximab (on CD20 of human B cells) is fundamentally different to antibodies that bind to the spike protein of the SAR-CoV2 virus? Cort's comment seems to suggest that the mabs' effects are similar:
It seems unlikely that this treatment would be any more effective at preventing post-Covid-19 ME/CFS than Covid-19 vaccinations given they achieve the same thing of anti-spike antibodies, except for people who are unable to make their own antibodies. I don't think we have seen evidence that people with ME/CFS are, on average, worse at making antibodies when confronted with a pathogen.
Edit to add - there is some evidence that Intermountain Healthcare may be legit. They are a not-for-profit hospital and their CEO featured in a New York Times article. Link here:How do we partner with Pharma?
Impact of Monoclonal Antibody Treatment on Post-Acute COVID-19 Syndrome (MAbPACs)
specifically casirivimab-imdevimab antibody infusion, anti-SARS-CoV-2 monoclonal antibodies that were given during acute Covid-19 treatment.
Summary: This treatment gives people the same sort of antibodies against SARS-CoV-2 that their bodies would produce if vaccinated. The trial aims to see if this treatment administered during the acute disease might reduce post-infection symptoms i.e. there it prevents any of the conditions lumped under the Long Covid name. In the trial design, there is a lack of differentiation between symptoms caused by various post-Covid conditions. Otherwise, it looks like an ok trial.
We know people still get post-Covid symptoms when they have SARS-CoV-2 antibodies from vaccinations, so, while this treatment might help, it's unlikely to have a massive impact on incidence.
This study is not trying to treat established Long Covid.
*****
Cort said:Now we’re talking. Monoclonal antibodies are all the rage in many diseases. These powerful and expensive drugs can target and turn off specific factors in the immune system. They’ve been talked about in ME/CFS, but except for a perhaps underpowered Rituximab trial, have never received a shot.
They’re going to get at least a preliminary shot here. This 260-person casirivimab-imdevimab trial at the Intermountain Medical Center in Murray, Utah used an antibody that worked well to both treat people infected with earlier variants and reduce the incidence of infection. This trial isn’t aiming to get rid of long COVID; instead, by giving it early in an infection, it’s trying to reduce the incidence of long COVID later.
If the trial succeeds, though, one might expect the RECOVER Initiative to do as it did with Paxlovid – and follow up with a trial in long COVID.
This retrospective study is being undertaken by Intermountain Healthcare in association with Regeneron Pharmaceuticals. It is wanting to see what impact giving a treatment during the acute illness that aims to reduce the risk of severe Covid-19 has on reporting of symptoms at the 4 to 5 month mark. Controls are to be matched on gender, age and Covid-19 risk.
According to Wikipedia, the casirivimab/imdevimab combo was only to be given to people who were at high risk for progression to severe Covid-19. It does sound as though these antibodies are useful in reducing the severity of the acute disease. As I understand it, these antibodies to the spike protein are of the sort that people would produce themselves when vaccinated, or during a Covid-19 infection.
The Clinical Trials entry says the study is not yet recruiting, although the start date was Aug 2021 and the expected end date was April 2022. So, I'm not sure how active the trial is.
The researchers don't seem to have a very good idea about the mix of health conditions that are bundled up in 'PACS' or Long Covid. There's talk about higher rates of Long Covid among people who are hospitalised, obese and have co-morbidities. It's a bit like doing a study of ME/CFS using a Fukuda diagnostic criteria, except that there is an even greater potential for heterogeneous cohorts. A mixed cohort potentially is ok for answering the specific question - 'does antibody treatment during the acute phase reduce post-Covid symptoms?', so long as researchers go into the study with their eyes open and are able to report on subgroups. I don't see any evidence of an awareness of the likely different symptom groups (e.g. ME/CFS; lung or heart damage), or any plans to group people with different types of post-Covid symptoms in the analysis. The primary outcome is a single 'Post-acute Covid-19 symptom score' - a number out of 60.
The secondary outcomes are have a lot of focus on mental health and on heath care costs e.g. number medically attended visits and health care costs between day 120 and day 150 after date of positive test; measures of depression, anxiety and post-traumatic stress disorder. There are some measures of quality of life and function.
I think this is a reasonable trial as far as it goes. I like that Intermountain Healthcare is making use of its database to evaluate the impact of an acute treatment on longer term outcomes. I don't see any evidence of them working with people who understand what Long Covid actually consists of. And I feel slightly concerned that the researchers will find that obese people with co-morbidities including depression and anxiety are more likely to report post-Covid symptoms and that it will be assumed that that finding applies also to people with post-Covid-19 ME/CFS.
If it's really true that vaccination does reduce the risk of post-Covid-19 ME/CFS (as opposed to just post-Covid symptoms in general), then I suppose that it's conceivable that this treatment (which supplies anti-spoke antibodies rather than inducing the person's body to make them itself) could also reduce the risk.
I'm not sure Cort fully understands what different monoclonal antibodies do. I don't know myself, but surely the action of Rituximab (on CD20 of human B cells) is fundamentally different to antibodies that bind to the spike protein of the SAR-CoV2 virus? Cort's comment seems to suggest that the mabs' effects are similar:
Cort said:Monoclonal antibodies are all the rage in many diseases. These powerful and expensive drugs can target and turn off specific factors in the immune system. They’ve been talked about in ME/CFS, but except for a perhaps underpowered Rituximab trial, have never received a shot.
Please correct me if I am wrong, but in the unlikely event that this treatment was found (in a later study) to help people with existing post-Covid-19 ME/CFS, then it would almost certainly mean that there was a reservoir of SARS-CoV-2 in the bodies of people with ME/CFS. If the ME/CFS is just a post-infection reaction, there's no obvious reason why antibodies to a pathogen that is no longer there would help.Cort said:If the trial succeeds, though, one might expect the RECOVER Initiative to do as it did with Paxlovid – and follow up with a trial in long COVID.
It seems unlikely that this treatment would be any more effective at preventing post-Covid-19 ME/CFS than Covid-19 vaccinations given they achieve the same thing of anti-spike antibodies, except for people who are unable to make their own antibodies. I don't think we have seen evidence that people with ME/CFS are, on average, worse at making antibodies when confronted with a pathogen.
Edit to add - there is some evidence that Intermountain Healthcare may be legit. They are a not-for-profit hospital and their CEO featured in a New York Times article. Link here:How do we partner with Pharma?
Last edited:
in the unlikely event that this treatment was found (in a later study) to help people with existing post-Covid-19 ME/CFS, then it would almost certainly mean that there was a reservoir of SARS-CoV-2 in the bodies of people with ME/CFS. If the ME/CFS is just a post-infection reaction, there's no obvious reason why antibodies to a pathogen that is no longer there would help.
Anti-idiotype Abs?
From A Possible Role for Anti-idiotype Antibodies in SARS-CoV-2 Infection and Vaccination (NEJM, 2022)
Every antibody that is induced and specific for an antigen (termed “Ab1” antibody) has immunogenic regions, particularly in their variable-region antigen-binding domains, that are unique as a result of genetic recombination of immunoglobulin variable, diversity, and joining (VDJ) genes; VDJ recombination results in new and therefore immunogenic amino acid sequences called idiotopes, which are then capable of inducing specific antibodies against Ab1 antibodies as a form of down-regulation. A similar paradigm has been proposed for T cells.
However, these regulatory immune responses are also capable of doing much more. The paratopes, or antigen-binding domains, of some of the resulting anti-idiotype (or “Ab2”) antibodies that are specific for Ab1 can structurally resemble that of the original antigens themselves. Thus, the Ab2 antigen-binding region can potentially represent an exact mirror image of the initial targeted antigen in the Ab1 response, and Ab2 antibodies have even been examined for potential use as a surrogate for the antigen in vaccine studies. However, as a result of this mimicry, Ab2 antibodies also have the potential to bind the same receptor that the original antigen was targeting. Ab2 antibodies binding to the original receptor on normal cells therefore have the potential to mediate profound effects on the cell that could result in pathologic changes, particularly in the long term — long after the original antigen itself has disappeared.
ETA: Although note Jo's comment from Nov 2021 here.
Last edited:
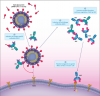
Picture from that NEJM paper that SNT Gatchaman linked - showing how the virus's spike protein can attach to the ACE2 receptor. And also showing that the anti-idiotype antibody that the body might make could also attach to the ACE2 receptor.
I can see how anti-idiotype antibodies might potentially cause a problem, perhaps enough of a problem to make ME/CFS. If for example the ongoing maintenance production of antibodies against pathogen antigens produces anti-idiotype antibodies, and those anti-idiotype antibodies act like the antigen or otherwise disrupt business as usual, that could be bad.
I don't know how pumping more exogenous antibodies (the mabs) into the body might prevent that process of making anti-idiotype antibodies though - the obvious possibility is that they might actually increase the odds of problematic anti-idiotype antibodies. I guess there are potential mechanisms with feedback loops.
But yeah, from the limited information, it seems like a reasonable study done by reasonable people. I'd just like to see a bit more of an indication that the researchers understand that Long Covid symptoms can be produced by a range of causes. Lumping people with PTSD and heart damage and lung damage and ME/CFS might obscure any benefits of the mabs.
From this useful source of info about Covid mabs,
https://www.idsociety.org/covid-19-...tics-and-interventions/monoclonal-antibodies/
the news that, due to the evolution of the Covid-19 virus, the casirivimab/imdevimab combo is no longer recommended to treat COVID-19 in the US.
The majority of direct antiviral monoclonal antibody products against COVID-19 target the SARS-CoV-2 spike protein, which the virus utilizes to enter host cells, thus blocking viral attachment and entry into human cells (Marovich, June 2020). Products that have at one point received FDA authorization include bamlanivimab/etesevimab, casirivimab/imdevimab (brand name REGEN-COV), sotrovimab and bebtelovimab. However, because the Omicron variant has become the dominant variant in the United States, and because emerging variants of Omicron have become resistant to the currently available monoclonals, all of these EUAs have been revoked, and there is no monoclonal antibody currently recommended for use to treat COVID-19.
